Bicuculline, pentobarbital and diazepam modulate spontaneous GABA(A) channels in rat hippocampal neurons
- PMID: 11030718
- PMCID: PMC1572380
- DOI: 10.1038/sj.bjp.0703621
Bicuculline, pentobarbital and diazepam modulate spontaneous GABA(A) channels in rat hippocampal neurons
Abstract
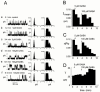
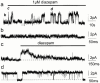
Spontaneously opening, chloride-selective channels that showed outward rectification were recorded in ripped-off patches from rat cultured hippocampal neurons and in cell-attached patches from rat hippocampal CA1 pyramidal neurons in slices. In both preparations, channels had multiple conductance states and the most common single-channel conductance varied. In the outside-out patches it ranged from 12 to 70 pS (Vp=40 mV) whereas in the cell-attached patches it ranged from 56 to 85 pS (-Vp=80 mV). Application of GABA to a patch showing spontaneous channel activity evoked a rapid, synchronous activation of channels. During prolonged exposure to either 5 or 100 microM GABA, the open probability of channels decreased. Application of GABA appeared to have no immediate effect on single-channel conductance. Exposure of the patches to 100 microM bicuculline caused a gradual decrease on the single-channel conductance of the spontaneous channels. The time for complete inhibition to take place was slower in the outside-out than in the cell-attached patches. Application of 100 microM pentobarbital or 1 microM diazepam caused 2 - 4 fold increase in the maximum channel conductance of low conductance (<40 pS) spontaneously active channels. The observation of spontaneously opening GABA(A) channels in cell-attached patches on neurons in slices suggests that they may have a role in neurons in vivo and could be an important site of action for some drugs such as benzodiazepines, barbiturates and general anaesthetics.
Figures





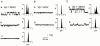

Similar articles
-
Pentobarbital modulates gamma-aminobutyric acid-activated single-channel conductance in rat cultured hippocampal neurons.Mol Pharmacol. 2000 Sep;58(3):463-9. Mol Pharmacol. 2000. PMID: 10953037
-
Conductance of GABAA channels activated by pentobarbitone in hippocampal neurons from newborn rats.J Physiol. 2003 Oct 1;552(Pt 1):13-22. doi: 10.1113/jphysiol.2003.047415. Epub 2003 Aug 1. J Physiol. 2003. PMID: 12897171 Free PMC article.
-
GABA concentration sets the conductance of delayed GABAA channels in outside-out patches from rat hippocampal neurons.J Membr Biol. 2001 Jun 1;181(3):171-83. doi: 10.1007/s00232-001-0021-5. J Membr Biol. 2001. PMID: 11420604
-
Activation and modulation of neuronal K+ channels by GABA.Trends Neurosci. 1992 Feb;15(2):46-51. doi: 10.1016/0166-2236(92)90025-4. Trends Neurosci. 1992. PMID: 1374961 Review.
-
Intracellular effectors and modulators of GABA-A and GABA-B receptors: a commentary.Biochimie. 1987 Apr;69(4):395-406. doi: 10.1016/0300-9084(87)90031-9. Biochimie. 1987. PMID: 2443189 Review.
Cited by
-
The regulatory role of GABAA receptor in Actinia equina nervous system and the possible effect of global ocean acidification.Pflugers Arch. 2021 Dec;473(12):1851-1858. doi: 10.1007/s00424-021-02628-w. Epub 2021 Oct 11. Pflugers Arch. 2021. PMID: 34633524 Free PMC article.
-
Intrinsic noise in cultured hippocampal neurons: experiment and modeling.J Neurosci. 2004 Oct 27;24(43):9723-33. doi: 10.1523/JNEUROSCI.1721-04.2004. J Neurosci. 2004. PMID: 15509761 Free PMC article.
-
GABAA receptors activate fish feeding behaviour via two distinct functional pathways.J Exp Biol. 2018 Feb 7;221(Pt 3):jeb170514. doi: 10.1242/jeb.170514. J Exp Biol. 2018. PMID: 29191862 Free PMC article.
-
GABA-independent GABAA receptor openings maintain tonic currents.J Neurosci. 2013 Feb 27;33(9):3905-14. doi: 10.1523/JNEUROSCI.4193-12.2013. J Neurosci. 2013. PMID: 23447601 Free PMC article.
-
GABA-activated single-channel and tonic currents in rat brain slices.J Vis Exp. 2011 Jul 17;(53):2858. doi: 10.3791/2858. J Vis Exp. 2011. PMID: 21788935 Free PMC article.
References
-
- BARNARD E.A., SKOLNICK P., OLSEN R.W., MOHLER H., SIEGHART W., BIGGIO G., BRAESTRUP C., BATESON A.N., LANGER S.Z. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol. Rev. 1998;50:291–313. - PubMed
-
- BIRNIR B., EGHBALI M., EVERITT A.B., COX G.B., GAGE P.W. Non-synaptic GABA-gated receptors exhibit great plasticity in channel conductance. Biophys. J. 1999;76:A338.
-
- BIRNIR B., EVERITT A.B., GAGE P.W. Characteristics of GABAA channels in rat dentate gyrus. J. Membrane. Biol. 1994;142:93–102. - PubMed
-
- BIRNIR B., EVERITT A.B., LIM M.S.F., GAGE P.W. Spontaneously opening GABAA channels in CA1 pyramidal neurones of rat hippocampus. J. Membrane Biol. 2000;174:21–29. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

