Analysis of 3-morpholinosydnonimine and sodium nitroprusside effects on dopamine release in the striatum of freely moving rats: role of nitric oxide, iron and ascorbic acid
- PMID: 11030735
- PMCID: PMC1572392
- DOI: 10.1038/sj.bjp.0703635
Analysis of 3-morpholinosydnonimine and sodium nitroprusside effects on dopamine release in the striatum of freely moving rats: role of nitric oxide, iron and ascorbic acid
Abstract
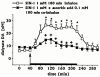
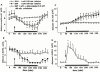
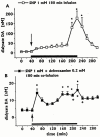
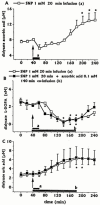
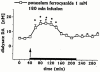
The effects of intrastriatal infusion of 3-morpholinosydnonimine (SIN-1) or sodium nitroprusside (SNP) on dopamine (DA), 3-methoxytyramine (3-MT), dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), L-dihydroxyphenylalanine (L-DOPA), ascorbic acid and uric acid concentrations in dialysates from the striatum of freely moving rats were evaluated using microdialysis. SIN-1 (1 mM) infusion for 180 min increased microdialysate DA and 3-MT concentrations, while L-DOPA, DOPCA+HVA, ascorbic acid and uric acid levels were unaffected. Co-infusion with ascorbic acid (0.1 mM) inhibited SIN-1-induced increases in DA and 3-MT dialysate concentration. SNP (1 mM) infusion for 180 min increased greatly the dialysate DA concentration to a peak (2950% of baseline) at the end of the infusion, while increases in 3-MT were negligible. In addition, SNP decreased ascorbic acid and L-DOPA but increased uric acid concentration in the dialysate. Co-infusion with deferoxamine (0.2 mM) inhibited the late SNP-induced increase in DA dialysate concentration, but did not affect the decrease in ascorbic acid and increase uric acid dialysate concentrations. SNP (1 mM) infusion for 20 min moderately increased uric acid, DA and 3-MT, but decreased L-DOPA levels in the dialysate. Ascorbic acid concentration increased at the end of SNP infusion. Co-infusion with ascorbic acid (0.1 mM) inhibited the SNP-induced increase in DA and 3-MT, but did not affect the decrease in L-DOPA and increase in uric acid dialysate concentrations. These results suggest that NO released from SIN-1 may account for the increase in the dialysate DA concentration. NO released following decomposition of SNP may account for the early increase in dialysate DA, while late changes in microdialysate composition following SNP may result from an interaction between NO and the ferrocyanide moiety of SNP. Exogenous ascorbic acid inhibits the effect of exogenous NO on DA release probably by scavenging NO, suggesting that endogenous ascorbic acid may modulate the NO control of DA release from 300 striatal dopaminergic terminals.
Figures

Similar articles
-
Analysis of S-nitroso-N-acetylpenicillamine effects on dopamine release in the striatum of freely moving rats: role of endogenous ascorbic acid and oxidative stress.Br J Pharmacol. 2001 Feb;132(4):941-9. doi: 10.1038/sj.bjp.0703887. Br J Pharmacol. 2001. PMID: 11181436 Free PMC article.
-
A study on the role of nitric oxide and iron in 3-morpholino-sydnonimine-induced increases in dopamine release in the striatum of freely moving rats.Br J Pharmacol. 2001 Sep;134(2):275-82. doi: 10.1038/sj.bjp.0704232. Br J Pharmacol. 2001. PMID: 11564645 Free PMC article.
-
Role of endogenous melatonin in the oxidative homeostasis of the extracellular striatal compartment: a microdialysis study in PC12 cells in vitro and in the striatum of freely moving rats.J Pineal Res. 2005 Nov;39(4):409-18. doi: 10.1111/j.1600-079X.2005.00266.x. J Pineal Res. 2005. PMID: 16207297
-
No nitric oxide for HO-1 from sodium nitroprusside.Mol Pharmacol. 2006 May;69(5):1507-9. doi: 10.1124/mol.106.023416. Epub 2006 Feb 13. Mol Pharmacol. 2006. PMID: 16476785 Review.
-
Rapid Dopamine Release in Freely Moving Rats.In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 2. In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 2. PMID: 21204389 Free Books & Documents. Review.
Cited by
-
Synthesis of Nitric Oxide Donors Derived from Piloty's Acid and Study of Their Effects on Dopamine Secretion from PC12 Cells.Pharmaceuticals (Basel). 2017 Sep 5;10(3):74. doi: 10.3390/ph10030074. Pharmaceuticals (Basel). 2017. PMID: 28872590 Free PMC article.
-
Analysis of S-nitroso-N-acetylpenicillamine effects on dopamine release in the striatum of freely moving rats: role of endogenous ascorbic acid and oxidative stress.Br J Pharmacol. 2001 Feb;132(4):941-9. doi: 10.1038/sj.bjp.0703887. Br J Pharmacol. 2001. PMID: 11181436 Free PMC article.
-
Does Vitamin C Influence Neurodegenerative Diseases and Psychiatric Disorders?Nutrients. 2017 Jun 27;9(7):659. doi: 10.3390/nu9070659. Nutrients. 2017. PMID: 28654017 Free PMC article. Review.
-
A study on the role of nitric oxide and iron in 3-morpholino-sydnonimine-induced increases in dopamine release in the striatum of freely moving rats.Br J Pharmacol. 2001 Sep;134(2):275-82. doi: 10.1038/sj.bjp.0704232. Br J Pharmacol. 2001. PMID: 11564645 Free PMC article.
References
-
- BATES J.N., BAKER M.T., GUERRA R., JR, HARRISON D.G. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochem. Pharmacol. 1991;42:S157–S165. - PubMed
-
- BECKER B.B. Towards the physiological function of uric acid. Free Rad. Biol. Med. 1993;14:615–631. - PubMed
-
- CACINI W. Comparative accumulation of uric acid and hypoxanthine by slices of avian renal cortex. J. Pharmacol. Exp. Ther. 1982;220:86–90. - PubMed
-
- COHEN A.M., ABERDROTH R.E., HOCHSTEIN P. Inhibition of free radical-induced DNA damage by uric acid. FEBS Lett. 1984;174:147–150. - PubMed
-
- DESOLE M.S., SCIOLA L., SIRCANA S., GODANI C., MIGHELI R., DELOGU M.R., PIRAS G., DE NATALE G., MIELE E. Protective effect of deferoxamine on sodium nitroprusside-induced apoptosis in PC12 cells. Neurosci. Lett. 1998;247:1–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

