Crystal structure of Streptococcus pneumoniae acyl carrier protein synthase: an essential enzyme in bacterial fatty acid biosynthesis
- PMID: 11032795
- PMCID: PMC314021
- DOI: 10.1093/emboj/19.20.5281
Crystal structure of Streptococcus pneumoniae acyl carrier protein synthase: an essential enzyme in bacterial fatty acid biosynthesis
Abstract
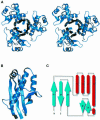
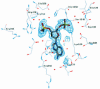
Acyl carrier protein synthase (AcpS) catalyzes the formation of holo-ACP, which mediates the essential transfer of acyl fatty acid intermediates during the biosynthesis of fatty acids and lipids in the cell. Thus, AcpS plays an important role in bacterial fatty acid and lipid biosynthesis, making it an attractive target for therapeutic intervention. We have determined, for the first time, the crystal structure of the Streptococcus pneumoniae AcpS and AcpS complexed with 3'5'-ADP, a product of AcpS, at 2.0 and 1.9 A resolution, respectively. The crystal structure reveals an alpha/beta fold and shows that AcpS assembles as a tightly packed functional trimer, with a non-crystallographic pseudo-symmetric 3-fold axis, which contains three active sites at the interface between protomers. Only two active sites are occupied by the ligand molecules. Although there is virtually no sequence similarity between the S.pneumoniae AcpS and the Bacillus subtilis Sfp transferase, a striking structural similarity between both enzymes was observed. These data provide a starting point for structure-based drug design efforts towards the identification of AcpS inhibitors with potent antibacterial activity.
Figures


References
-
- Badger J., Kumar,R.A., Yip,P. and Szalma,S. (1999) New features and enhancements in the X-PLOR computer program. Proteins, 35, 25–33. - PubMed
-
- Brünger A.T. (1992) The free R value: a novel statistical quantity for assessing the accuracy of crystal structures. Nature, 355, 472–474. - PubMed
-
- Carreras C.W., Gehring,A.M., Walsh,C.T. and Khosla,C. (1997) Utilization of enzymatically phosphopantetheinylated acyl carrier proteins and acetyl-acyl carrier proteins by the actinorhodin polyketide synthase. Biochemistry, 36, 11757–11761. - PubMed
-
- Cohen M.L. (1992) Epidemiology of drug resistance: implications for a postantimicrobial era. Science, 257, 1050–1055. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

