Importin-11, a nuclear import receptor for the ubiquitin-conjugating enzyme, UbcM2
- PMID: 11032817
- PMCID: PMC314000
- DOI: 10.1093/emboj/19.20.5502
Importin-11, a nuclear import receptor for the ubiquitin-conjugating enzyme, UbcM2
Abstract
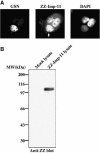
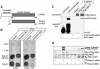
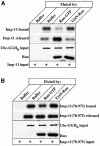
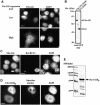
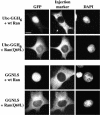
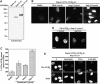
Importins are members of a family of transport receptors (karyopherins) that mediate the nucleocytoplasmic transport of protein and RNA cargoes. We identified importin-11 as a potential new human member of this family, on the basis of limited similarity to the Saccharomyces cerevisiae protein, Lph2p, and cloned the complete open reading frame. Importin-11 interacts with the Ran GTPase, and constitutively shuttles between the nuclear and cytoplasmic compartments. A yeast dihybrid screen identified UbcM2, an E2-type ubiquitin-conjugating enzyme, as a binding partner and potential transport cargo for importin-11. Importin-11 and UbcM2 interact directly, and the complex is disassembled by Ran:GTP but not by Ran:GDP. UbcM2 is constitutively nuclear and shuttles between the nuclear and cytoplasmic compartments. Nuclear import of UbcM2 requires Ran and importin-11, and is inhibited by wheatgerm agglutinin, energy depletion or dominant interfering mutants of Ran and importin-beta. These data establish importin-11 as a new member of the karyopherin family of transport receptors, and identify UbcM2 as a nuclear member of the E2 ubiquitin-conjugating enzyme family.
Figures


References
-
- Borer R.A., Lehner,C.F., Eppenberger,H.M. and Nigg,E.A. (1989) Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell, 56, 379–390. - PubMed
-
- Chen P., Johnson,P., Sommer,T., Jentsch,S. and Hochstrasser,M. (1993) Multiple ubiquitin-conjugating enzymes participate in the in vivo degradation of the yeast MATα2 repressor. Cell, 74, 357–369. - PubMed
-
- Chook Y.M. and Blobel,G. (1999) Structure of the nuclear transport complex karyopherin-2-Ran.GppNHp. Nature, 399, 230–237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

