Pax3 is required for enteric ganglia formation and functions with Sox10 to modulate expression of c-ret
- PMID: 11032856
- PMCID: PMC314346
- DOI: 10.1172/JCI10828
Pax3 is required for enteric ganglia formation and functions with Sox10 to modulate expression of c-ret
Abstract
Hirschsprung disease and Waardenburg syndrome are human genetic diseases characterized by distinct neural crest defects. Patients with Hirschsprung disease suffer from gastrointestinal motility disorders, whereas Waardenburg syndrome consists of defective melanocyte function, deafness, and craniofacial abnormalities. Mutations responsible for Hirschsprung disease and Waardenburg syndrome have been identified, and some patients have been described with characteristics of both disorders. Here, we demonstrate that PAX3, which is often mutated in Waardenburg syndrome, is required for normal enteric ganglia formation. Pax3 can bind to and activate expression of the c-RET gene, which is often mutated in Hirschsprung disease. Pax3 functions with Sox10 to activate transcription of c-RET, and SOX10 mutations result in Waardenburg-Hirschsprung syndrome. Thus, Pax3, Sox10, and c-Ret are components of a neural crest development pathway, and interruption of this pathway at various stages results in neural crest-related human genetic syndromes.
Figures

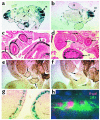
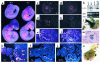
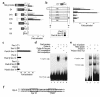
References
-
- LeDouarin, N.M. 1982. The neural crest. Cambridge University Press. London, United Kingdom. 259 pp.
-
- McKusick, V.A. 1998. Online mendelian inheritance in man, OMIM™. http://www.ncbi.nlm.nih.gov/Omim. Johns Hopkins University and National Center for Biotechnology Information, National Library of Medicine. Baltimore, Maryland, USA, and Bethesda, Maryland, USA.
-
- Gershon MD. Disorders of enteric neuronal development: insights from transgenic mice. Am J Physiol. 1999;277:G262–G267. - PubMed
-
- Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119:1005–1017. - PubMed
-
- Romeo G, et al. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung’s disease. Nature. 1994;367:377–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

