Apoptosis control in syncytia induced by the HIV type 1-envelope glycoprotein complex: role of mitochondria and caspases
- PMID: 11034598
- PMCID: PMC2195869
- DOI: 10.1084/jem.192.8.1081
Apoptosis control in syncytia induced by the HIV type 1-envelope glycoprotein complex: role of mitochondria and caspases
Abstract
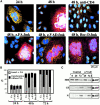
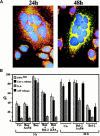
Syncytia arising from the fusion of cells expressing a lymphotropic HIV type 1-encoded envelope glycoprotein complex (Env) with cells expressing the CD4/CXC chemokine receptor 4 complex spontaneously undergo cell death. Here we show that this process is accompanied by caspase activation and signs of mitochondrial membrane permeabilization (MMP), including the release of intermembrane proteins such as cytochrome c (Cyt-c) and apoptosis-inducing factor (AIF) from mitochondria. In Env-induced syncytia, caspase inhibition did not suppress AIF- and Cyt-c translocation, yet it prevented all signs of nuclear apoptosis. Translocation of Bax to mitochondria led to MMP, which was inhibited by microinjected Bcl-2 protein or bcl-2 transfection. Bcl-2 also prevented the subsequent nuclear chromatin condensation and DNA fragmentation. The release of AIF occurred before that of Cyt-c and before caspase activation. Microinjection of AIF into syncytia sufficed to trigger rapid, caspase-independent Cyt-c release. Neutralization of endogenous AIF by injection of an antibody prevented all signs of spontaneous apoptosis occurring in syncytia, including the Cyt-c release and nuclear apoptosis. In contrast, Cyt-c neutralization only prevented nuclear apoptosis, and did not affect AIF release. Our results establish that the following molecular sequence governs apoptosis of Env-induced syncytia: Bax-mediated/Bcl-2-inhibited MMP --> AIF release --> Cyt-c release --> caspase activation --> nuclear apoptosis.
Figures

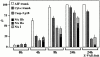



References
-
- Fauci A.S. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. - PubMed
-
- Gougeon M.L., Montagnier L. Programmed cell death as a mechanism of CD4 and CD8 T cell depletion in AIDS. Molecular control and effect of highly active anti-retroviral therapy. Ann. NY Acad. Sci. 1999;887:199–212. - PubMed
-
- Li C.J., Friedman D.J., Wang C., Metelev V., Pardee A.B. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science. 1995;268:429–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

