Nuclear factor kappa B is required for the development of marginal zone B lymphocytes
- PMID: 11034607
- PMCID: PMC2195875
- DOI: 10.1084/jem.192.8.1175
Nuclear factor kappa B is required for the development of marginal zone B lymphocytes
Abstract
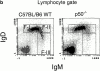
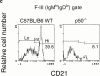
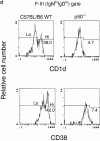
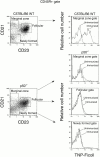
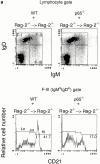
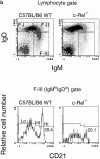
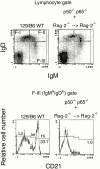
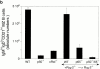
Although immunoglobulin (Ig)M(hi)IgD(lo/-)CD21(hi) marginal zone B cells represent a significant proportion of naive peripheral splenic B lymphocytes, few of the genes that regulate their development have been identified. This subset of peripheral B cells fails to emerge in mice that lack nuclear factor (NF)-kappa Bp50. Less drastic reductions in marginal zone B cell numbers are also seen in the spleens of recombination activating gene (Rag)-2(-/-) mice reconstituted with NF-kappa Bp65(-/-) fetal liver cells and in c-Rel(-/-) mice. In contrast, steady-state levels of IgD(hi) splenic follicular B cells are not significantly reduced in the absence of NF-kappa Bp50, NF-kappa Bp65, or c-Rel. Reconstitution of B cells in Rag-2(-/-) mice with a mixture of p50(-/-)/p65(-/-) fetal liver cells and Rag-2(-/-) bone marrow cells revealed that the generation of marginal zone B cells requires the expression of NF-kappa B in developing B cells, as opposed to supporting cells.
Figures


References
-
- MacLennan I.C.M., Gray D. Antigen driven selection of virgin and memory B cells. Immunol. Rev. 1986;91:61–85. - PubMed
-
- Kraal G. Cells in the marginal zone of the spleen. Int. Rev. Cytol. 1992;132:31–74. - PubMed
-
- Oliver A.M., Martin F., Gartland G.L., Carter R.H., Kearney J.F. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur. J. Immunol. 1997;27:2366–2374. - PubMed
-
- Oliver A.M., Martin F., Kearney J.F. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J. Immunol. 1999;162:7198–7207. - PubMed
-
- Makowska A., Faizunnessa N.N., Anderson P., Midtvedt T., Cardell S. CD1high B cellsa population of mixed origin. Eur. J. Immunol. 1999;29:3285–3295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

