Protein kinase C-dependent modulation of Na+ currents increases the excitability of rat neocortical pyramidal neurones
- PMID: 11034619
- PMCID: PMC2270127
- DOI: 10.1111/j.1469-7793.2000.00291.x
Protein kinase C-dependent modulation of Na+ currents increases the excitability of rat neocortical pyramidal neurones
Abstract
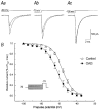
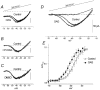
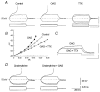
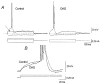
The effect of the protein kinase C (PKC) activator 1-oleoyl-2-acetyl-sn-glycerol (OAG) on TTX-sensitive Na+ currents in neocortical pyramidal neurones was evaluated using voltage-clamp and intracellular current-clamp recordings. In pyramid-shaped dissociated neurones, the addition of OAG to the superfusing medium consistently led to a 30% reduction in the maximal peak amplitude of the transient sodium current (I(Na,T)) evoked from a holding potential of -70 mV. We attributed this inhibitory effect to a significant negative shift of the voltage dependence of steady-state channel inactivation (of approximately 14 mV). The inhibitory effect was completely prevented by hyperpolarising prepulses to potentials that were more negative than -80 mV. A small but significant leftward shift of INa,T activation was also observed, resulting in a slight increase of the currents evoked by test pulses at potentials more negative then -35 mV. In the presence of OAG, the activation of the persistent fraction of the Na+ current (INa,P) evoked by means of slow ramp depolarisations was consistently shifted in the negative direction by 3.9+/-0.5 mV, while the peak amplitude of the current was unaffected. In slice experiments, the OAG perfusion enhanced a subthreshold depolarising rectification affecting the membrane response to the injection of positive current pulses, and thus led the neurones to fire in response to significantly lower depolarising stimuli than those needed under control conditions. This effect was attributed to an OAG-induced enhancement of INa,P, since it was observed in the same range of potentials over which I(Na,P) activates and was completely abolished by TTX. The qualitative firing characteristics of both the intrinsically bursting and regular spiking neurones were unaffected when OAG was added to the physiological perfusing medium, but their firing frequency increased in response to slight suprathreshold depolarisations. The obtained results suggest that physiopathological events working through PKC activation can increase neuronal excitability by directly amplifying the I(Na,P)-dependent subthreshold depolarisation, and that this facilitating effect may override the expected reduction in neuronal excitability deriving from OAG-induced inhibition of the maximal INa, T peak amplitude.
Figures






Similar articles
-
Anemone toxin (ATX II)-induced increase in persistent sodium current: effects on the firing properties of rat neocortical pyramidal neurones.J Physiol. 1998 Feb 15;507 ( Pt 1)(Pt 1):105-16. doi: 10.1111/j.1469-7793.1998.105bu.x. J Physiol. 1998. PMID: 9490824 Free PMC article.
-
PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade.J Physiol. 1996 Sep 1;495 ( Pt 2)(Pt 2):429-40. doi: 10.1113/jphysiol.1996.sp021604. J Physiol. 1996. PMID: 8887754 Free PMC article.
-
Protein-kinase C-dependent phosphorylation inhibits the effect of the antiepileptic drug topiramate on the persistent fraction of sodium currents.Neuroscience. 2004;127(1):63-8. doi: 10.1016/j.neuroscience.2004.04.040. Neuroscience. 2004. PMID: 15219669
-
Modulation of cardiac Na+ channels expressed in a mammalian cell line and in ventricular myocytes by protein kinase C.Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3289-93. doi: 10.1073/pnas.91.8.3289. Proc Natl Acad Sci U S A. 1994. PMID: 8159741 Free PMC article.
-
Voltage-dependent conductances of solitary ganglion cells dissociated from the rat retina.J Physiol. 1987 Apr;385:361-91. doi: 10.1113/jphysiol.1987.sp016497. J Physiol. 1987. PMID: 2443669 Free PMC article. Review.
Cited by
-
Activation of 5-HT2A receptors by TCB-2 induces recurrent oscillatory burst discharge in layer 5 pyramidal neurons of the mPFC in vitro.Physiol Rep. 2014 May 20;2(5):e12003. doi: 10.14814/phy2.12003. Print 2014 May 1. Physiol Rep. 2014. PMID: 24844635 Free PMC article.
-
Gradient of sodium current across the left ventricular wall of adult rat hearts.J Physiol. 2001 Oct 15;536(Pt 2):439-43. doi: 10.1111/j.1469-7793.2001.0439c.xd. J Physiol. 2001. PMID: 11600679 Free PMC article.
-
Protein kinase C activation mediates interferon-β-induced neuronal excitability changes in neocortical pyramidal neurons.J Neuroinflammation. 2014 Oct 29;11:185. doi: 10.1186/s12974-014-0185-4. J Neuroinflammation. 2014. PMID: 25359459 Free PMC article.
-
Modulation of voltage-gated sodium channels hyperpolarizes the voltage threshold for activation in spinal motoneurones.Exp Brain Res. 2012 Mar;217(2):311-22. doi: 10.1007/s00221-011-2994-3. Epub 2012 Jan 5. Exp Brain Res. 2012. PMID: 22218500
-
Astrocytes expressing mutant SOD1 and TDP43 trigger motoneuron death that is mediated via sodium channels and nitroxidative stress.Front Cell Neurosci. 2014 Feb 7;8:24. doi: 10.3389/fncel.2014.00024. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24570655 Free PMC article.
References
-
- Akiyama K, Ono M, Kohira I, Daigen A, Ishihara T, Kuroda S. Long-lasting increase in protein kinase C activity in the hippocampus of amygdala kindled rat. Brain Research. 1995;679:212–230. - PubMed
-
- Astman N, Gutnick MJ, Fleidervish IA. Activation of protein kinase C increases neuronal excitability by regulating persistent Na+ current in mouse neocortical slices. Journal of Neurophysiology. 1998;80:1547–1551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

