Modulation of B-cell proliferative response by a soluble extract of Nippostrongylus brasiliensis
- PMID: 11035719
- PMCID: PMC97693
- DOI: 10.1128/IAI.68.11.6154-6161.2000
Modulation of B-cell proliferative response by a soluble extract of Nippostrongylus brasiliensis
Abstract
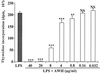
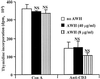
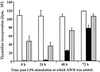
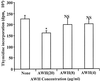
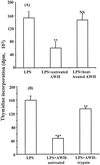
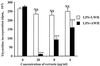
We and others have previously shown that nematodes or nematode products can stimulate or inhibit the generation of lymphocyte responses, suggesting that nematodes exert diverse effects on the developing immune responses of their host. In this study we examined the immunomodulatory effect of a soluble extract of Nippostrongylus brasiliensis (adult worm homogenate [AWH]) on B-cell responsiveness. We found that the extract inhibited the proliferation of B cells to lipopolysaccharide (LPS) stimulation in a dose-dependent manner. This effect was specific to B cells, since the extract did not inhibit T-cell proliferation to concanavalin A or anti-CD3 stimulation. The data presented here confirm that the extract is not toxic to B cells. We present evidence that the active factor is proteinaceous in nature and that the inhibitory activity is restricted to the adult stage of Nb. The extract does not appear to interfere with early activation events since it can be added up to 48 h after LPS stimulation, and it inhibited responses to phorbol myristate acetate and ionomycin. Furthermore, the proliferation of B cells to other activators was also inhibited by AWH. This observation shows that the inhibitory activity of AWH is not restricted to LPS-mediated B-cell proliferation. We present evidence that, in the absence of accessory cells, the inhibitory effect of the extract was ablated. This observation shows that the activity of AWH is not mediated directly on B cells but is mediated via the production of negative signals from accessory cells (macrophages), which affect a downstream pathway required by all B-cell activators tested. These effects on B-cell and accessory cell function are likely to have a significant effect on the outcome of infections experienced concurrently.
Figures



Similar articles
-
Nematode infection enhances survival of activated T cells by modulating accessory cell function.J Immunol. 1999 Nov 1;163(9):5005-12. J Immunol. 1999. PMID: 10528205
-
Extract of Nippostrongylus brasiliensis stimulates polyclonal type-2 immunoglobulin response by inducing De novo class switch.Infect Immun. 2000 Sep;68(9):4913-22. doi: 10.1128/IAI.68.9.4913-4922.2000. Infect Immun. 2000. PMID: 10948105 Free PMC article.
-
IL-2 inhibits IL-4-dependent IgE and IgG1 production in vitro and in vivo.Int Immunol. 1995 Feb;7(2):259-68. doi: 10.1093/intimm/7.2.259. Int Immunol. 1995. PMID: 7734421
-
Immunomodulation by nematodes: a review.Vet Parasitol. 1984 Jun;14(3-4):299-320. doi: 10.1016/0304-4017(84)90098-0. Vet Parasitol. 1984. PMID: 6382784 Review.
-
Studies on B-cell activation in vitro.Ann Immunol (Paris). 1983 Jul-Aug;134D(1):63-73. doi: 10.1016/s0769-2625(83)80057-9. Ann Immunol (Paris). 1983. PMID: 6354068 Review.
Cited by
-
Modulation of a heterologous immune response by the products of Ascaris suum.Infect Immun. 2002 Nov;70(11):6058-67. doi: 10.1128/IAI.70.11.6058-6067.2002. Infect Immun. 2002. PMID: 12379682 Free PMC article.
-
Helicobacter pylori infection in an urban African population.J Clin Microbiol. 2001 Apr;39(4):1323-7. doi: 10.1128/JCM.39.4.1323-1327.2001. J Clin Microbiol. 2001. PMID: 11283050 Free PMC article.
-
Induction of murine immune responses by DNA encoding a 23-kDa antigen of Cryptosporidium parvum.Parasitol Res. 2007 Sep;101(4):943-50. doi: 10.1007/s00436-007-0565-0. Epub 2007 May 9. Parasitol Res. 2007. PMID: 17487508
References
-
- Albina J E, Abate J A, Henry W., Jr Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation: role of IFN-γ in the induction of the nitric oxide synthesizing pathway. J Immunol. 1991;147:144–148. - PubMed
-
- Allen J E, Lawrence R A, Maizels R M. APC from mice harbouring the filarial nematode, Brugia malayi, prevents cellular proliferation but not cytokine production. Int Immunol. 1996;8:143–151. - PubMed
-
- Allen J E, Macdonald A S. Profound suppression of cellular proliferation mediated by the secretions of nematodes. Parasite Immunol. 1998;20:241–247. - PubMed
-
- Armitage R J, Alderson M R. B cell stimulation. Curr Opin Immunol. 1995;7:243–247. - PubMed
-
- Bouchard C, Fridman W H, Sautè C. Mechanism of inhibition of lipopolysaccharide-stimulated mouse B cell responses by transforming growth factor-β1. Immunol Lett. 1994;40:105–110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

