Lack of CD4(+) T cells does not affect induction of CD8(+) T-cell immunity against Encephalitozoon cuniculi infection
- PMID: 11035729
- PMCID: PMC97703
- DOI: 10.1128/IAI.68.11.6223-6232.2000
Lack of CD4(+) T cells does not affect induction of CD8(+) T-cell immunity against Encephalitozoon cuniculi infection
Abstract
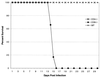
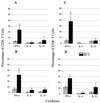
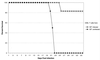
Cell-mediated immunity has been reported to play an important role in defense against Encephalitozoon cuniculi infection. Previous studies from our laboratory have underlined the importance of cytotoxic CD8(+) T lymphocytes (CTL) in survival of mice infected with E. cuniculi. In the present study, immune response against E. cuniculi infection in CD4(+) T-cell-deficient mice was evaluated. Similar to resistant wild-type animals, CD4(-/-) mice were able to resolve E. cuniculi infection even at a very high challenge dose (5 x 10(7) spores/mouse). Tissues from infected CD4(-/-) mice did not exhibit higher parasite loads in comparison to the parental wild-type mice. Conversely, at day 21 postinfection, susceptible CD8(-/-) mice had 10(14) times more parasites in the liver compared to control wild-type mice. Induction of the CD8(+) T-cell response in CD4(-/-) mice against E. cuniculi infection was studied. Interestingly, a normal antigen-specific CD8(+) T-cell response to E. cuniculi infection was observed in CD4(-/-) mice (precursor proliferation frequency, 1/2.5 x 10(4) versus 1/10(4) in wild-type controls). Lack of CD4(+) T cells did not alter the magnitude of the antigen-specific CTL response (precursor CTL frequency; 1/1.4 x 10(4) in CD4(-/-) mice versus 1/3 x 10(4) in control mice). Adoptive transfer of immune CD8(+) T cells from both CD4(-/-) and wild-type animals prevented the mortality in CD8(-/-) mice. E. cuniculi infection thus offers an example of an intracellular parasitic infection where CD8(+) T-cell immunity can be induced in the absence of CD4(+) T cells.
Figures





Similar articles
-
Gamma delta T cell-deficient mice have a down-regulated CD8+ T cell immune response against Encephalitozoon cuniculi infection.J Immunol. 2001 Jun 15;166(12):7389-97. doi: 10.4049/jimmunol.166.12.7389. J Immunol. 2001. PMID: 11390490
-
Induction of a rapid and strong antigen-specific intraepithelial lymphocyte response during oral Encephalitozoon cuniculi infection.J Immunol. 2004 Apr 1;172(7):4402-9. doi: 10.4049/jimmunol.172.7.4402. J Immunol. 2004. PMID: 15034055 Free PMC article.
-
Pure CD4+ T lymphocytes fail to protect perorally infected SCID mice from lethal microsporidiosis caused by Encephalitozoon cuniculi.Parasitol Res. 2006 Nov;99(6):682-6. doi: 10.1007/s00436-006-0208-x. Parasitol Res. 2006. PMID: 16738893
-
Immune response to Encephalitozoon cuniculi infection.Microbes Infect. 2001 Apr;3(5):401-5. doi: 10.1016/s1286-4579(01)01397-1. Microbes Infect. 2001. PMID: 11369277 Free PMC article. Review.
-
[Host immune response in mammals to Encephalitozoon cuniculi infection].Epidemiol Mikrobiol Imunol. 2004 Aug;53(3):136-41. Epidemiol Mikrobiol Imunol. 2004. PMID: 15524273 Review. Slovak.
Cited by
-
Latent microsporidiosis caused by Encephalitozoon cuniculi in immunocompetent hosts: a murine model demonstrating the ineffectiveness of the immune system and treatment with albendazole.PLoS One. 2013 Apr 11;8(4):e60941. doi: 10.1371/journal.pone.0060941. Print 2013. PLoS One. 2013. PMID: 23593356 Free PMC article.
-
Novel synthetic polyamines are effective in the treatment of experimental microsporidiosis, an opportunistic AIDS-associated infection.Antimicrob Agents Chemother. 2002 Jan;46(1):55-61. doi: 10.1128/AAC.46.1.55-61.2002. Antimicrob Agents Chemother. 2002. PMID: 11751111 Free PMC article.
-
Deficient humoral responses underlie susceptibility to Toxoplasma gondii in CD4-deficient mice.Infect Immun. 2002 Jan;70(1):185-91. doi: 10.1128/IAI.70.1.185-191.2002. Infect Immun. 2002. PMID: 11748181 Free PMC article.
-
Immune Response to Microsporidia.Exp Suppl. 2022;114:373-388. doi: 10.1007/978-3-030-93306-7_13. Exp Suppl. 2022. PMID: 35544009
-
Effector CD8 T cell immunity in microsporidial infection: a lone defense mechanism.Semin Immunopathol. 2015 May;37(3):281-7. doi: 10.1007/s00281-015-0482-8. Epub 2015 Apr 10. Semin Immunopathol. 2015. PMID: 25860800 Free PMC article. Review.
References
-
- Binder D, Kundig T M. Antiviral protection by CD8+ versus CD4+ T cells. CD8+ T cells correlating with cytotoxic activity in vitro are more efficient in antivaccinia virus protection than CD4-dependent IL. J Immunol. 1991;146:4301–4307. - PubMed
-
- Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. - PubMed
-
- Cali A, Takvorian P M, Lewin S, Rendel M, Sian C S, Wittner M, Tanowitz H B, Keohane E, Weiss L M. Brachiola vesicularum, n. g., n. sp., a new microsporidium associated with AIDS and myositis. J Eukaryot Microbiol. 1998;45:240–251. - PubMed
-
- Canning E, Lom J. The microsporidia of vertebrates. New York, N.Y: Academic Press; 1986.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

