Staphylococcal fibronectin binding protein interacts with heat shock protein 60 and integrins: role in internalization by epithelial cells
- PMID: 11035741
- PMCID: PMC97715
- DOI: 10.1128/IAI.68.11.6321-6328.2000
Staphylococcal fibronectin binding protein interacts with heat shock protein 60 and integrins: role in internalization by epithelial cells
Abstract
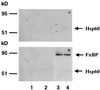
We reported previously that internalization of Staphylococcus aureus by nonprofessional phagocytes involves an interaction between fibronectin (Fn) binding protein (FnBP) and the host cell, resulting in signal transduction, tyrosine kinase activity, and cytoskeletal rearrangement (K. Dziewanowska, J. M. Patti, C. F. Deobald, K. W. Bayles, W. R. Trumble, and G. A. Bohach, Infect. Immun. 67:4673-4678, 1999). The goal of the present study was to identify the host molecules responsible for uptake of the organism through an interaction with FnBP. First, Fn was required for internalization. Addition of small amounts of exogenous Fn stimulated the uptake of S. aureus by HEp-2 cells, which are deficient in Fn synthesis. Fn antibodies blocked internalization of the organism by MAC-T cell monolayers, a bovine epithelial cell line which expresses Fn. Second, a monoclonal antibody (MAb) specific for beta(1) integrins dramatically reduced S. aureus invasion, suggesting that the formation of a Fn bridge linking the host cell beta(1) integrin and FnBP precedes internalization. However, ligand blotting of cell membrane proteins with a functional fragment of FnBP consistently identified an additional approximately 55-kDa receptor on both human and bovine epithelial cells. This protein was purified and identified by N-terminal microsequencing as heat shock protein 60 (Hsp60). The interaction between FnBP and Hsp60 also occurred when the whole cells were used. Cell membrane localization of Hsp60 was confirmed by biotinylation with an agent nonpermeable to the cell membrane. Pretreatment of epithelial cells with a MAb specific for eukaryotic Hsp60 significantly reduced internalization of S. aureus. Combined, these results suggest that the FnBP binds directly to both Hsp60 and Fn and is linked to beta(1) integrins through a Fn bridge. The simultaneous involvement of Fn and two host cell ligands, beta(1) integrins and Hsp60, suggests that FnBP is a multifunctional adhesin that mediates internalization in a manner similar to that proposed for OpaA, the Neisseria gonorrhoeae FnBP homolog (J. P. M. van Putten, T. D. Duensing, and R. L. Cole, Mol. Microbiol. 29:369-379, 1998).
Figures






References
-
- Azem A, Kessel M, Goloubinoff P. Characterization of a functional GroEL14(GroES7)2 chaperonin hetero-oligomer. Science. 1994;265:653–656. - PubMed
-
- Berendt A R, McCormick C J. Use of host adhesion molecules by infectious agents. In: Leendert P C, Issekutz T B, editors. Adhesion molecules in health and disease. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 347–379.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

