SclA, a novel collagen-like surface protein of Streptococcus pyogenes
- PMID: 11035747
- PMCID: PMC97721
- DOI: 10.1128/IAI.68.11.6370-6377.2000
SclA, a novel collagen-like surface protein of Streptococcus pyogenes
Abstract
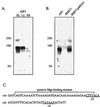
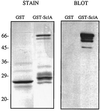
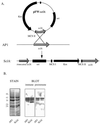
Surface proteins of Streptococcus pyogenes are important virulence factors. Here we describe a novel collagen-like surface protein, designated SclA (streptococcal collagen-like surface protein). The sclA gene was identified in silico using the Streptococcal Genome Sequencing Project with the recently identified protein GRAB as the probe. SclA has a signal sequence and a cell wall attachment region containing the prototypic LPXTGX motif. The surface-exposed part of SclA contains a unique NH(2)-terminal domain of 73 amino acids, followed by a collagen-like region. The sclA gene was found to be positively regulated by Mga, a transcriptional activator of several S. pyogenes virulence determinants. A mutant lacking cell wall-associated SclA was constructed and was found to be as effective as wild-type bacteria in platelet aggregation, survival in fresh human blood, and adherence to pharyngeal cells. The sclA gene was found in all 12 S. pyogenes strains that were investigated using PCR. Sequence analysis revealed that the signal sequence and the cell wall attachment region are highly conserved. The collagen-like domain is variable in its NH(2)-terminal region and has conserved repeated domains in its COOH-terminal part. SclA proteins from most strains have additional proline-rich repeats spacing the collagen-like domain and the cell wall attachment sequence. The unique NH(2)-terminal region is hypervariable, but computer predictions indicate a common secondary structure, with two alpha helices connected by a loop region. Immune selection may explain the hypervariability in the NH(2)-terminal region, whereas the preserved secondary structure implies that this region has a common function. These features and the Mga regulation are shared with the M protein of S. pyogenes. Moreover, as with the gene encoding the M protein, phylogenetic analysis indicates that horizontal gene transfer has contributed to the evolution of sclA.
Figures


Similar articles
-
Transcriptional activation of sclA by Mga requires a distal binding site in Streptococcus pyogenes.J Bacteriol. 2004 Dec;186(23):7847-57. doi: 10.1128/JB.186.23.7847-7857.2004. J Bacteriol. 2004. PMID: 15547255 Free PMC article.
-
Unique regulation of SclB - a novel collagen-like surface protein of Streptococcus pyogenes.Mol Microbiol. 2001 Jun;40(6):1427-38. doi: 10.1046/j.1365-2958.2001.02493.x. Mol Microbiol. 2001. PMID: 11442840
-
Streptococcus pyogenes sclB encodes a putative hypervariable surface protein with a collagen-like repetitive structure.Microbiology (Reading). 2001 Feb;147(Pt 2):419-429. doi: 10.1099/00221287-147-2-419. Microbiology (Reading). 2001. PMID: 11158359
-
Structure, function, and genetics of streptococcal M protein.Rev Infect Dis. 1988 Jul-Aug;10 Suppl 2:S356-9. doi: 10.1093/cid/10.supplement_2.s356. Rev Infect Dis. 1988. PMID: 3055203 Review.
-
The spatial regulation of protein sorting in Streptococcus pyogenes.2022 Jul 17 [updated 2022 Sep 3]. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]. 2nd edition. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2022 Oct 8. Chapter 5. 2022 Jul 17 [updated 2022 Sep 3]. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]. 2nd edition. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2022 Oct 8. Chapter 5. PMID: 36479772 Free Books & Documents. Review.
Cited by
-
The group A streptococcal collagen-like protein-1, Scl1, mediates biofilm formation by targeting the extra domain A-containing variant of cellular fibronectin expressed in wounded tissue.Mol Microbiol. 2013 Feb;87(3):672-89. doi: 10.1111/mmi.12125. Epub 2012 Dec 26. Mol Microbiol. 2013. PMID: 23217101 Free PMC article.
-
Multicenter Clinical Evaluation of the Novel Alere i Strep A Isothermal Nucleic Acid Amplification Test.J Clin Microbiol. 2015 Jul;53(7):2258-61. doi: 10.1128/JCM.00490-15. Epub 2015 May 13. J Clin Microbiol. 2015. PMID: 25972418 Free PMC article.
-
Transcriptional activation of sclA by Mga requires a distal binding site in Streptococcus pyogenes.J Bacteriol. 2004 Dec;186(23):7847-57. doi: 10.1128/JB.186.23.7847-7857.2004. J Bacteriol. 2004. PMID: 15547255 Free PMC article.
-
Rgg influences the expression of multiple regulatory loci to coregulate virulence factor expression in Streptococcus pyogenes.Infect Immun. 2002 Feb;70(2):762-70. doi: 10.1128/IAI.70.2.762-770.2002. Infect Immun. 2002. PMID: 11796609 Free PMC article.
-
Adaptation of the group A Streptococcus adhesin Scl1 to bind fibronectin type III repeats within wound-associated extracellular matrix: implications for cancer therapy.Mol Microbiol. 2019 Sep;112(3):800-819. doi: 10.1111/mmi.14317. Epub 2019 Jun 12. Mol Microbiol. 2019. PMID: 31145503 Free PMC article.
References
-
- Åkesson P, Cooney J, Kishimoto F, Björck L. Protein H—a novel IgG binding bacterial protein. Mol Immunol. 1990;6:523–531. - PubMed
-
- Åkesson P, Sjöholm A G, Björck L. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J Biol Chem. 1996;271:1081–1088. - PubMed
-
- Berge A, Sjöbring U. PAM, a novel plasminogen-binding protein from Streptococcus pyogenes. J Biol Chem. 1993;268:25417–25424. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

