Temporal pore formation-mediated egress from macrophages and alveolar epithelial cells by Legionella pneumophila
- PMID: 11035756
- PMCID: PMC97730
- DOI: 10.1128/IAI.68.11.6431-6440.2000
Temporal pore formation-mediated egress from macrophages and alveolar epithelial cells by Legionella pneumophila
Abstract
Legionella pneumophila does not induce apoptosis in the protozoan host, but induces pore formation-mediated cytolysis after termination of intracellular replication (L.-Y. Gao and Y. Abu Kwaik, Environ. Microbiol. 2:79-90, 2000). In contrast to this single mode of killing of protozoa, we have recently proposed a biphasic model by which L. pneumophila kills macrophages, in which the first phase is manifested through the induction of apoptosis during early stages of the infection, followed by an independent and temporal induction of necrosis during late stages of intracellular replication. Here we show that, similar to the protozoan host, the induction of necrosis and cytolysis of macrophages by L. pneumophila is mediated by the pore-forming toxin or activity. This activity is temporally and maximally expressed only upon termination of bacterial replication and correlates with cytolysis of macrophages and alveolar epithelial cells in vitro. We have identified five L. pneumophila mutants defective in the pore-forming activity. The phagosomes harboring the mutants do not colocalize with the late endosomal or lysosomal marker Lamp-1, and the mutants replicate intracellularly similar to the parental strain. Interestingly, despite their prolific intracellular replication, the mutants are defective in cytotoxicity and are "trapped" within and fail to lyse and egress from macrophages and alveolar epithelial cells upon termination of intracellular replication. However, the mutants are subsequently released from the host cell, most likely due to apoptotic death of the host cell. Data derived from cytotoxicity assays, confocal laser scanning microscopy, and electron microscopy confirm the defect in the mutants to induce necrosis of macrophages and the failure to egress from the host cell. Importantly, the mutants are completely defective in acute lethality (24 to 48 h) to intratracheally inoculated A/J mice. We conclude that the pore-forming activity of L. pneumophila is not required for phagosomal trafficking or for intracellular replication. This activity is expressed upon termination of bacterial replication and is essential to induce cytolysis of infected macrophages to allow egress of intracellular bacteria. In addition, this activity plays a major role in pulmonary immunopathology in vivo.
Figures
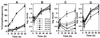
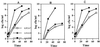


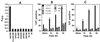

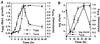

References
-
- Abu Kwaik Y. Fatal attraction of mammalian cells to Legionella pneumophila. Mol Microbiol. 1998;30:689–696. - PubMed
-
- Abu Kwaik Y, Engleberg N C. Cloning and molecular characterization of a Legionella pneumophila gene induced by intracellular infection and by various in vitro stress stimuli. Mol Microbiol. 1994;13:243–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

