Expression and characterization of recombinant human-derived Pneumocystis carinii dihydrofolate reductase
- PMID: 11036028
- PMCID: PMC101608
- DOI: 10.1128/AAC.44.11.3092-3096.2000
Expression and characterization of recombinant human-derived Pneumocystis carinii dihydrofolate reductase
Abstract
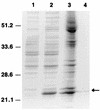
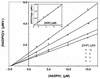
Dihydrofolate reductase (DHFR) is the target of trimethoprim (TMP), which has been widely used in combination with sulfa drugs for treatment and prophylaxis of Pneumocystis carinii pneumonia. While the rat-derived P. carinii DHFR has been well characterized, kinetic studies of human-derived P. carinii DHFR, which differs from rat-derived P. carinii DHFR by 38% in amino acid sequence, have not been reported to date. Here we report on the expression and kinetic characterization of the recombinant human-derived P. carinii DHFR. The 618-bp coding sequence of the human-derived P. carinii DHFR gene was expressed in Escherichia coli. As determined by sodium dodecyl sulfate-polyacrylamide gel eletrophoresis, the purified enzyme had a molecular mass of 25 kDa, consistent with that predicted from the DNA sequence. Kinetic analysis showed that the K(m) values for dihydrofolate and NADPH were 2.7 +/- 0.3 and 14.0 +/- 4.3 microM, respectively, which are similar to those reported for rat-derived P. carinii DHFR. Inhibition studies revealed that both TMP and pyrimethamine were poor inhibitors of human-derived P. carinii DHFR, with K(i) values of 0.28 +/- 0.08 and 0.065 +/- 0.005 microM, respectively, while trimetrexate and methotrexate were potent inhibitors, with K(i) values of 0.23 +/- 0.03 and 0.016 +/- 0.004 nM, respectively. The availability of purified recombinant enzyme in large quantities should facilitate the identification of antifolate inhibitors with greater potency and higher selectivity for human-derived P. carinii DHFR.
Figures
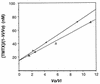
References
-
- Allegra C J, Chabner B A, Tuazon C U, Ogata-Arakaki D, Baird B, Drake J C, Simmons J T, Lack E E, Shelhamer J H, Balis F, et al. Trimetrexate for the treatment of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1987;317:978–985. - PubMed
-
- Baccanari D P, Joyner S S. Dihydrofolate reductase hysteresis and its effect of inhibitor binding analyses. Biochemistry. 1981;20:1710–1716. - PubMed
-
- Blakley R L. Eukaryotic dihydrofolate reductase. Adv Enzymol Relat Areas Mol Biol. 1995;70:23–102. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

