Transgenic mice expressing a mutant form of loricrin reveal the molecular basis of the skin diseases, Vohwinkel syndrome and progressive symmetric erythrokeratoderma
- PMID: 11038186
- PMCID: PMC2192631
- DOI: 10.1083/jcb.151.2.401
Transgenic mice expressing a mutant form of loricrin reveal the molecular basis of the skin diseases, Vohwinkel syndrome and progressive symmetric erythrokeratoderma
Abstract
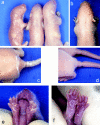
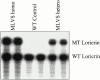
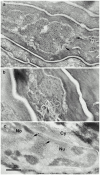
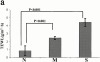
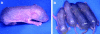
Mutations in the cornified cell envelope protein loricrin have been reported recently in some patients with Vohwinkel syndrome (VS) and progressive symmetric erythrokeratoderma (PSEK). To establish a causative relationship between loricrin mutations and these diseases, we have generated transgenic mice expressing a COOH-terminal truncated form of loricrin that is similar to the protein expressed in VS and PSEK patients. At birth, transgenic mice (ML.VS) exhibited erythrokeratoderma with an epidermal barrier dysfunction. 4 d after birth, high-expressing transgenic animals showed a generalized scaling of the skin, as well as a constricting band encircling the tail and, by day 7, a thickening of the footpads. Histologically, ML. VS transgenic mice also showed retention of nuclei in the stratum corneum, a characteristic feature of VS and PSEK. Immunofluorescence and immunoelectron microscopy showed the mutant loricrin protein in the nucleus and cytoplasm of epidermal keratinocytes, but did not detect the protein in the cornified cell envelope. Transfection experiments indicated that the COOH-terminal domain of the mutant loricrin contains a nuclear localization signal. To determine whether the ML.VS phenotype resulted from dominant-negative interference of the transgene with endogenous loricrin, we mated the ML.VS transgenics with loricrin knockout mice. A severe phenotype was observed in mice that lacked expression of wild-type loricrin. Since loricrin knockout mice are largely asymptomatic (Koch, P.K., P. A. de Viragh, E. Scharer, D. Bundman, M.A. Longley, J. Bickenbach, Y. Kawachi, Y. Suga, Z. Zhou, M. Huber, et al., J. Cell Biol. 151:389-400, this issue), this phenotype may be attributed to expression of the mutant form of loricrin. Thus, deposition of the mutant protein in the nucleus appears to interfere with late stages of epidermal differentiation, resulting in a VS-like phenotype.
Figures
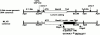








Comment in
-
The complexity and redundancy of epithelial barrier function.J Cell Biol. 2000 Oct 16;151(2):F5-8. doi: 10.1083/jcb.151.2.f5. J Cell Biol. 2000. PMID: 11038193 Free PMC article. No abstract available.
References
-
- Armstrong D.K.B., McKenna K.E., Hughes A.E. A novel insertional mutation in loricrin in Vohwinkel's keratoderma. J. Invest. Dermatol. 1998;111:702–704. - PubMed
-
- Boulikas T. Nuclear localization signals (NLS) Crit. Rev. Eukaryot. Gene Expr. 1993;3:193–227. - PubMed
-
- Camisa C., Rossana C. Variant of keratoderma hereditaria mutilans (Vohwinkel's syndrome). Treatment with orally administered isotretinoin. Arch. Dermatol. 1984;120:1323–1328. - PubMed
-
- Dingwall C., Laskey R.A. Nuclear targeting sequence—a consensus? Trends. Biochem. Sci. 1991;16:478–481. - PubMed
-
- DiSepio D., Jones A., Longley M.A., Bundman D., Rothnagel J.A., Roop D.R. The proximal promoter of the mouse loricrin gene contains a functional AP-1 element and directs keratinocyte-specific but not differentiation-specific expression. J. Biol. Chem. 1995;270:10792–10799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

