HIV-1 membrane fusion: targets of opportunity
- PMID: 11038194
- PMCID: PMC2192632
- DOI: 10.1083/jcb.151.2.f9
HIV-1 membrane fusion: targets of opportunity
Figures
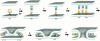

Comment on
-
Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion.J Cell Biol. 2000 Oct 16;151(2):413-23. doi: 10.1083/jcb.151.2.413. J Cell Biol. 2000. PMID: 11038187 Free PMC article.
-
The transmembrane domain of influenza hemagglutinin exhibits a stringent length requirement to support the hemifusion to fusion transition.J Cell Biol. 2000 Oct 16;151(2):425-37. doi: 10.1083/jcb.151.2.425. J Cell Biol. 2000. PMID: 11038188 Free PMC article.
References
-
- Bullough P.A., Hughson F.M., Skehel J.J., Wiley D.C. Structure of influenza hemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. - PubMed
-
- Chan D.C., Fass D., Berger J.M., Kim P.S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. - PubMed
-
- Chan D.C., Kim P.S. HIV entry and its inhibition. Cell. 1998;93:681–684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

