Jasmonate is essential for insect defense in Arabidopsis
- PMID: 11038546
- PMCID: PMC24703
- DOI: 10.1073/pnas.94.10.5473
Jasmonate is essential for insect defense in Arabidopsis
Abstract
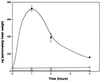
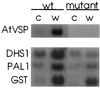
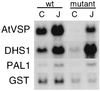
The signaling pathways that allow plants to mount defenses against chewing insects are known to be complex. To investigate the role of jasmonate in wound signaling in Arabidopsis and to test whether parallel or redundant pathways exist for insect defense, we have studied a mutant (fad3-2 fad7-2 fad8) that is deficient in the jasmonate precursor linolenic acid. Mutant plants contained negligible levels of jasmonate and showed extremely high mortality ( approximately 80%) from attack by larvae of a common saprophagous fungal gnat, Bradysia impatiens (Diptera: Sciaridae), even though neighboring wild-type plants were largely unaffected. Application of exogenous methyl jasmonate substantially protected the mutant plants and reduced mortality to approximately 12%. These experiments precisely define the role of jasmonate as being essential for the induction of biologically effective defense in this plant-insect interaction. The transcripts of three wound-responsive genes were shown not to be induced by wounding of mutant plants but the same transcripts could be induced by application of methyl jasmonate. By contrast, measurements of transcript levels for a gene encoding glutathione S-transferase demonstrated that wound induction of this gene is independent of jasmonate synthesis. These results indicate that the mutant will be a good genetic model for testing the practical effectiveness of candidate defense genes.
Figures

References
-
- Ryan C A. Annu Rev Phytopathol. 1990;28:425–449.
-
- Bowles D J. Annu Rev Biochem. 1990;59:873–908. - PubMed
-
- Baldwin I T. In: Insect–Plant Interactions. Bernays E A, editor. Boca Raton, FL: CRC; 1994. pp. 1–23.
-
- Hilder V A, Gatehouse A M R, Sheerman S E, Barker R F, Boulter D. Nature (London) 1987;330:160–163.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

