RecA-like proteins are components of early meiotic nodules in lily
- PMID: 11038554
- PMCID: PMC21251
- DOI: 10.1073/pnas.94.13.6868
RecA-like proteins are components of early meiotic nodules in lily
Abstract
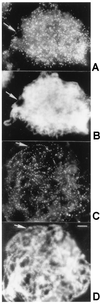
Early meiotic nodules (also called recombination nodules) are proteinaceous structures about 100 nm in diameter that are associated with forming synaptonemal complexes (SCs) during early prophase I of meiosis. Although their function is unknown, early nodules may be involved in searching for DNA homology before synaptic initiation. Two potential components of early nodules are Rad51 and Dmc1 proteins. These proteins are important for meiotic recombination in eukaryotes and are homologous to RecA, the major protein that catalyzes homologous pairing and DNA strand exchange in prokaryotes. In addition, Rad51 has been localized by immunofluorescence in abundant foci that may correspond to early nodules in yeast, lily, and mouse. In yeast and lily, Dmc1 and Lim15, the lily homolog of Dmc1, colocalize with Rad51. Here, using electron microscopic immunogold localization to spreads of zygotene and early pachytene SCs from lily, we confirm that RecA-like proteins are components of early nodules. The antibody used was generated to full-length tomato Rad51 protein and binds to both Rad51 and Lim15 in immunoblots of lily primary microsporocyte proteins. The labeled early nodules are heterogeneous in size and are associated with both axial elements and SCs. There are two classes of early nodules, those that are densely labeled with gold and those that are not labeled at all. This result may be due to technical limitations associated with using spread preparations or to differences in the nodules themselves. The presence of Rad51 and/or Lim15 proteins in early nodules supports the hypothesis that early nodules are involved in recombination-related events during meiosis.
Figures



References
-
- Moses M. Annu Rev Genet. 1968;2:363–412.
-
- Gillies C B. CRC Crit Rev Plant Sci. 1984;2:81–116.
-
- von Wettstein D, Rasmussen S W, Holm P B. Annu Rev Genet. 1984;18:331–413. - PubMed
-
- Carpenter A T C. In: Genetic Recombination. Kucherlapati R, Smith G R, editors. Washington, DC: Am. Soc. Microbiol.; 1988. pp. 529–548.
LinkOut - more resources
Full Text Sources
Research Materials

