Purified vesicles of tobacco cell vacuolar and plasma membranes exhibit dramatically different water permeability and water channel activity
- PMID: 11038555
- PMCID: PMC21292
- DOI: 10.1073/pnas.94.13.7103
Purified vesicles of tobacco cell vacuolar and plasma membranes exhibit dramatically different water permeability and water channel activity
Abstract
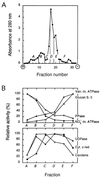
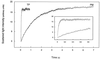
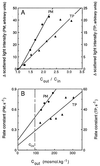
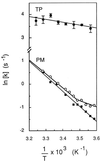
The vacuolar membrane or tonoplast (TP) and the plasma membrane (PM) of tobacco suspension cells were purified by free-flow electrophoresis (FFE) and aqueous two-phase partitioning, with enrichment factors from a crude microsomal fraction of >/=4- to 5-fold and reduced contamination by other cellular membranes. For each purified fraction, the mean apparent diameter of membrane vesicles was determined by freeze-fracture electron microscopy, and the osmotic shrinking kinetics of the vesicles were characterized by stopped-flow light scattering. Osmotic water permeability coefficients (Pf) of 6.1 +/- 0.2 and 7.6 +/- 0.9 microm . s(-1) were deduced for PM-enriched vesicles purified by FFE and phase partitioning, respectively. The associated activation energies (Ea; 13.7 +/- 1.0 and 13.4 +/- 1.4 kcal . mol(-1), respectively) suggest that water transport in the purified PM occurs mostly by diffusion across the lipid matrix. In contrast, water transport in TP vesicles purified by FFE was characterized by (i) a 100-fold higher Pf of 690 +/- 35 microm . s(-1), (ii) a reduced Ea of 2.5 +/- 1.3 kcal . mol(-1), and (iii) a reversible inhibition by mercuric chloride, up to 83% at 1 mM. These results provide functional evidence for channel-mediated water transport in the TP, and more generally in a higher plant membrane. A high TP Pf suggests a role for the vacuole in buffering osmotic fluctuations occurring in the cytoplasm. Thus, the differential water permeabilities and water channel activities observed in the tobacco TP and PM point to an original osmoregulatory function for water channels in relation to the typical compartmentation of plant cells.
Figures

References
-
- Steudle E. In: Flux Control in Bological Systems: From Enzymes to Populations and Ecosystems. Schultze E D, editor. San Diego: Academic; 1994. pp. 237–299.
-
- Finkelstein A. Distinguished Lecture Series of the Society of General Physiologists. Vol. 4. New York: Wiley; 1987. pp. 1–228.
-
- Url W G. Protoplasma. 1971;72:427–447.
-
- Collander R. In: Plant Physiology: A Treatise. Steward F C, editor. New York and London: Academic; 1959. pp. 3–102.
-
- Zimmermann U. Methods Enzymol. 1989;174:338–366.
LinkOut - more resources
Full Text Sources
Other Literature Sources

