In vitro reconstitution of the photosystem I light-harvesting complex LHCI-730: heterodimerization is required for antenna pigment organization
- PMID: 11038558
- PMCID: PMC23880
- DOI: 10.1073/pnas.94.14.7667
In vitro reconstitution of the photosystem I light-harvesting complex LHCI-730: heterodimerization is required for antenna pigment organization
Abstract
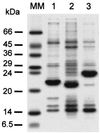
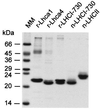
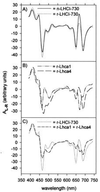
Here we describe the in vitro reconstitution of photosystem I light-harvesting complexes with pigments and proteins (Lhca1 and Lhca4) obtained by overexpression of tomato Lhca genes in Escherichia coli. Using Lhca1 and Lhca4 individually for reconstitution results in monomeric pigment-proteins, whereas a combination thereof yields a dimeric complex. Interactions of the apoproteins is highly specific, as reconstitution of either of the two constituent proteins in combination with a light-harvesting protein of photosystem II does not result in dimerization. The reconstituted Lhca1/4, but not complexes obtained with either Lhca1 or Lhca4 alone, closely resembles the native LHCI-730 dimer from tomato leaves with regard to spectroscopic properties, pigment composition, and stoichiometry. Monomeric complexes of Lhca1 or Lhca4 possess lower pigment/protein ratios, indicating that interactions of the two subunits not only facilitates pigment reorganization but also recruitment of additional pigments. In addition to higher averages of chlorophyll a/b ratios in monomeric complexes than in LHCI-730, comparative fluorescence and CD spectra demonstrate that heterodimerization involves preferential ligation of more chlorophyll b.
Figures


Similar articles
-
Identification of N- and C-terminal amino acids of Lhca1 and Lhca4 required for formation of the heterodimeric peripheral photosystem I antenna LHCI-730.Biochemistry. 2002 Jul 23;41(29):9126-31. doi: 10.1021/bi016042x. Biochemistry. 2002. PMID: 12119027
-
The Lhca antenna complexes of higher plants photosystem I.Biochim Biophys Acta. 2002 Oct 3;1556(1):29-40. doi: 10.1016/s0005-2728(02)00304-3. Biochim Biophys Acta. 2002. PMID: 12351216
-
Protein domains required for formation of stable monomeric Lhca1- and Lhca4-complexes.Photosynth Res. 2000;63(3):217-24. doi: 10.1023/A:1006499517613. Photosynth Res. 2000. PMID: 16228432
-
Excitation decay pathways of Lhca proteins: a time-resolved fluorescence study.J Phys Chem B. 2005 Nov 10;109(44):21150-8. doi: 10.1021/jp0519316. J Phys Chem B. 2005. PMID: 16853740
-
Light harvesting in photosystem I supercomplexes.Biochemistry. 2006 Jan 17;45(2):331-45. doi: 10.1021/bi051932o. Biochemistry. 2006. PMID: 16401064 Review.
Cited by
-
Energy transfer pathways in the minor antenna complex CP29 of photosystem II: a femtosecond study of carotenoid to chlorophyll transfer on mutant and WT complexes.Biophys J. 2003 Apr;84(4):2517-32. doi: 10.1016/S0006-3495(03)75057-7. Biophys J. 2003. PMID: 12668460 Free PMC article.
-
Structural and functional organization of the peripheral light-harvesting system in photosystem I.Photosynth Res. 2005;85(1):33-50. doi: 10.1007/s11120-004-6474-5. Photosynth Res. 2005. PMID: 15977058 Review.
-
Light-harvesting features revealed by the structure of plant photosystem I.Photosynth Res. 2004;81(3):239-50. doi: 10.1023/B:PRES.0000036881.23512.42. Photosynth Res. 2004. PMID: 16034530
-
Reconstitution of the peridinin-chlorophyll a protein (PCP): evidence for functional flexibility in chlorophyll binding.Photosynth Res. 2005 Nov;86(1-2):229-40. doi: 10.1007/s11120-005-2067-1. Photosynth Res. 2005. PMID: 16172941
-
The properties of the chlorophyll a/b-binding proteins Lhca2 and Lhca3 studied in vivo using antisense inhibition.Plant Physiol. 2001 Sep;127(1):150-8. doi: 10.1104/pp.127.1.150. Plant Physiol. 2001. PMID: 11553743 Free PMC article.
References
-
- Green B R, Durnford D G. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:685–714. - PubMed
-
- Haworth P, Watson J L, Arntzen C J. Biochim Biophys Acta. 1983;724:151–158.
-
- Schwartz E, Shen D, Aebersold R, McGrath J M, Pichersky E, Green B R. FEBS Lett. 1991;280:229–234. - PubMed
-
- Jansson S. Biochim Biophys Acta. 1994;1184:1–19. - PubMed
-
- Wolfe G R, Cunningham F X, Jr, Durnford D, Green B R, Gantt E. Nature (London) 1994;367:566–568.
LinkOut - more resources
Full Text Sources

