Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation
- PMID: 11040213
- PMCID: PMC316999
- DOI: 10.1101/gad.834100
Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation
Abstract
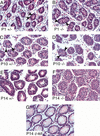
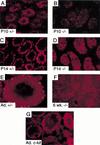
The only molecular similarity in sex determination found so far among phyla is between the Drosophila doublesex (dsx) and Caenorhabditis elegans mab-3 genes. dsx and mab-3 contain a zinc finger-like DNA-binding motif called the DM domain, perform several related regulatory functions, and are at least partially interchangeable in vivo. A DM domain gene called Dmrt1 has been implicated in male gonad development in a variety of vertebrates, on the basis of embryonic expression and chromosomal location. Such evidence is highly suggestive of a conserved role(s) for Dmrt1 in vertebrate sexual development, but there has been no functional analysis of this gene in any species. Here we show that murine Dmrt1 is essential for postnatal testis differentiation, with mutant phenotypes similar to those caused by human chromosome 9p deletions that remove the gene. As in the case of 9p deletions, Dmrt1 is dispensable for ovary development in the mouse. Thus, as in invertebrates, a DM domain gene regulates vertebrate male development.
Figures




References
-
- Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol. 1996;6:298–304. - PubMed
-
- Calvari V, Bertini V, De Grandi A, Peverali G, Zuffardi O, Ferguson-Smith M, Knudtzon J, Camerino G, Borsani G, Guioli S. A new submicroscopic deletion that refines the 9p region for sex reversal. Genomics. 2000;65:203–212. - PubMed
-
- Coschigano KT, Wensink PC. Sex-specific transcriptional regulation by male and female doublesex proteins of Drosophila. Genes & Dev. 1993;7:42–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
