Simulation of fungal-mediated cell death by fumonisin B1 and selection of fumonisin B1-resistant (fbr) Arabidopsis mutants
- PMID: 11041878
- PMCID: PMC149121
- DOI: 10.1105/tpc.12.10.1811
Simulation of fungal-mediated cell death by fumonisin B1 and selection of fumonisin B1-resistant (fbr) Arabidopsis mutants
Abstract
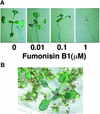
Fumonisin B1 (FB1), a programmed cell death-eliciting toxin produced by the necrotrophic fungal plant pathogen Fusarium moniliforme, was used to simulate pathogen infection in Arabidopsis. Plants infiltrated with 10 microM FB1 and seedlings transferred to agar media containing 1 microM FB1 develop lesions reminiscent of the hypersensitive response, including generation of reactive oxygen intermediates, deposition of phenolic compounds and callose, accumulation of phytoalexin, and expression of pathogenesis-related (PR) genes. Arabidopsis FB1-resistant (fbr) mutants were selected directly by sowing seeds on agar containing 1 microM FB1, on which wild-type seedlings fail to develop. Two mutants chosen for further analyses, fbr1 and fbr2, had altered PR gene expression in response to FB1. fbr1 and fbr2 do not exhibit differential resistance to the avirulent bacterial pathogen Pseudomonas syringae pv maculicola (ES4326) expressing the avirulence gene avrRpt2 but do display enhanced resistance to a virulent isogenic strain that lacks the avirulence gene. Our results demonstrate the utility of FB1 for high-throughput isolation of Arabidopsis defense-related mutants and suggest that pathogen-elicited programmed cell death of host cells may be an important feature of compatible plant-pathogen interactions.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

