Cell cycle regulation of the tobacco ribonucleotide reductase small subunit gene is mediated by E2F-like elements
- PMID: 11041892
- PMCID: PMC149135
Cell cycle regulation of the tobacco ribonucleotide reductase small subunit gene is mediated by E2F-like elements
Abstract
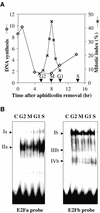
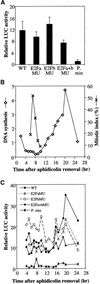
Ribonucleotide reductase (RNR) is a key enzyme involved in the DNA synthesis pathway. The RNR-encoded genes are cell cycle regulated and specifically expressed in S phase. The promoter of the RNR2 gene encoding for the small subunit was isolated from tobacco. Both in vivo and in vitro studies of the DNA-protein interactions in synchronized BY2 tobacco cells showed that two E2F-like motifs were involved in multiple specific complexes, some of which displayed cell cycle-regulated binding activities. Moreover, these two elements could specifically interact with a purified tobacco E2F protein. Involvement of the E2F elements in regulating the RNR2 promoter was checked by functional analyses in synchronized transgenic BY2 cells transformed with various RNR2 promoter constructs fused to the luciferase reporter gene. The two E2F elements were involved in upregulation of the promoter at the G1/S transition and mutation of both elements prevented any significant induction of the RNR promoter. In addition, one of the E2F elements sharing homology with the animal E2F/cell cycle-dependent element motif behaved like a repressor when outside of the S phase. These data provide evidence that E2F elements play a crucial role in cell cycle regulation of gene transcription in plants.
Figures




References
-
- An, G. (1987). Binary Ti vectors for plant transformation and promoter analysis. Methods Enzymol. 153, 292–305.
-
- Beijersbergen, R.L., Kerhoven, R.M., Zhu, L., Carlee, L., Voorhoeve, P.M., and Bernards, R. (1994). E2F–4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev. 8, 2680–2690. - PubMed
-
- Chabouté, M.-E., Combettes, B., Clément, B., Gigot, C., and Philipps, G. (1998). Molecular characterization of tobacco ribonucleotide reductase RNR1 and RNR2 cDNAs and cell cycle–regulated expression in synchronized plant cells. Plant Mol. Biol. 38, 797–806. - PubMed
-
- Chaubet, N., Flénet, M., Clément, B., Brignon, P., and Gigot, C. (1996). Identification of cis-elements regulating the expression of an Arabidopsis histone H4 gene. Plant J. 10, 425–435. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
