Intracellular localization of vaccinia virus extracellular enveloped virus envelope proteins individually expressed using a Semliki Forest virus replicon
- PMID: 11044098
- PMCID: PMC110928
- DOI: 10.1128/jvi.74.22.10535-10550.2000
Intracellular localization of vaccinia virus extracellular enveloped virus envelope proteins individually expressed using a Semliki Forest virus replicon
Abstract
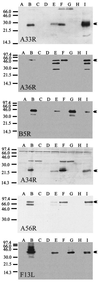
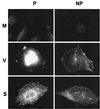
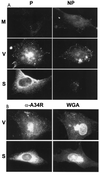
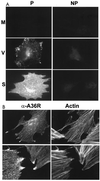
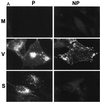
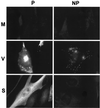
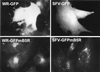
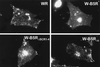
The extracellular enveloped virus (EEV) form of vaccinia virus is bound by an envelope which is acquired by wrapping of intracellular virus particles with cytoplasmic vesicles containing trans-Golgi network markers. Six virus-encoded proteins have been reported as components of the EEV envelope. Of these, four proteins (A33R, A34R, A56R, and B5R) are glycoproteins, one (A36R) is a nonglycosylated transmembrane protein, and one (F13L) is a palmitylated peripheral membrane protein. During infection, these proteins localize to the Golgi complex, where they are incorporated into infectious virus that is then transported and released into the extracellular medium. We have investigated the fates of these proteins after expressing them individually in the absence of vaccinia infection, using a Semliki Forest virus expression system. Significant amounts of proteins A33R and A56R efficiently reached the cell surface, suggesting that they do not contain retention signals for intracellular compartments. In contrast, proteins A34R and F13L were retained intracellularly but showed distributions different from that of the normal infection. Protein A36R was partially retained intracellularly, decorating both the Golgi complex and structures associated with actin fibers. A36R was also transported to the plasma membrane, where it accumulated at the tips of cell projections. Protein B5R was efficiently targeted to the Golgi region. A green fluorescent protein fusion with the last 42 C-terminal amino acids of B5R was sufficient to target the chimeric protein to the Golgi region. However, B5R-deficient vaccinia virus showed a normal localization pattern for other EEV envelope proteins. These results point to the transmembrane or cytosolic domain of B5R protein as one, but not the only, determinant of the retention of EEV proteins in the wrapping compartment.
Figures







Similar articles
-
The cytoplasmic and transmembrane domains of the vaccinia virus B5R protein target a chimeric human immunodeficiency virus type 1 glycoprotein to the outer envelope of nascent vaccinia virions.J Virol. 1997 Apr;71(4):3178-87. doi: 10.1128/JVI.71.4.3178-3187.1997. J Virol. 1997. PMID: 9060681 Free PMC article.
-
Mapping and functional analysis of interaction sites within the cytoplasmic domains of the vaccinia virus A33R and A36R envelope proteins.J Virol. 2003 Apr;77(7):4113-26. doi: 10.1128/jvi.77.7.4113-4126.2003. J Virol. 2003. PMID: 12634370 Free PMC article.
-
Golgi network targeting and plasma membrane internalization signals in vaccinia virus B5R envelope protein.J Virol. 2000 Apr;74(8):3771-80. doi: 10.1128/jvi.74.8.3771-3780.2000. J Virol. 2000. PMID: 10729152 Free PMC article.
-
The formation and function of extracellular enveloped vaccinia virus.J Gen Virol. 2002 Dec;83(Pt 12):2915-2931. doi: 10.1099/0022-1317-83-12-2915. J Gen Virol. 2002. PMID: 12466468 Review.
-
The exit of vaccinia virus from infected cells.Virus Res. 2004 Dec;106(2):189-97. doi: 10.1016/j.virusres.2004.08.015. Virus Res. 2004. PMID: 15567497 Review.
Cited by
-
Generation and characterization of a large panel of murine monoclonal antibodies against vaccinia virus.Virology. 2011 Jan 20;409(2):271-9. doi: 10.1016/j.virol.2010.10.019. Epub 2010 Nov 5. Virology. 2011. PMID: 21056889 Free PMC article.
-
Characterization and use of mammalian-expressed vaccinia virus extracellular membrane proteins for quantification of the humoral immune response to smallpox vaccines.Clin Vaccine Immunol. 2007 Aug;14(8):1032-44. doi: 10.1128/CVI.00050-07. Epub 2007 Jun 27. Clin Vaccine Immunol. 2007. PMID: 17596428 Free PMC article.
-
Predicting the subcellular localization of viral proteins within a mammalian host cell.Virol J. 2006 Apr 4;3:24. doi: 10.1186/1743-422X-3-24. Virol J. 2006. PMID: 16595001 Free PMC article.
-
Vaccinia Virus Glycoproteins A33, A34, and B5 Form a Complex for Efficient Endoplasmic Reticulum to trans-Golgi Network Transport.J Virol. 2020 Mar 17;94(7):e02155-19. doi: 10.1128/JVI.02155-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31941777 Free PMC article.
-
Protein B5 is required on extracellular enveloped vaccinia virus for repulsion of superinfecting virions.J Gen Virol. 2012 Sep;93(Pt 9):1876-1886. doi: 10.1099/vir.0.043943-0. Epub 2012 May 23. J Gen Virol. 2012. PMID: 22622330 Free PMC article.
References
-
- Appleyard G, Andrews C. Neutralizing activities of antisera to poxvirus soluble antigens. J Gen Virol. 1974;23:197–200. - PubMed
-
- Appleyard G, Hapel A J, Boulter E A. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen Virol. 1971;13:9–17. - PubMed
-
- Berglund P, Sjoberg M, Garoff H, Atkins G J, Sheahan B J, Liljestrom P. Semliki Forest virus expression system: production of conditionally infectious recombinant particles. Bio/Technology. 1993;11:916–920. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

