Development of multigene and regulated lentivirus vectors
- PMID: 11044103
- PMCID: PMC110933
- DOI: 10.1128/jvi.74.22.10589-10599.2000
Development of multigene and regulated lentivirus vectors
Abstract
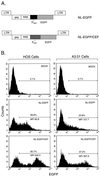
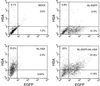
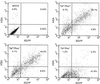
Previously we described safe and efficient three-component human immunodeficiency virus type 1 (HIV-1)-based gene transfer systems for delivery of genes into nondividing cells (H. Mochizuki, J. P. Schwartz, K. Tanaka, R. O. Brady, and J. Reiser, J. Virol. 72:8873-8883, 1998). To apply such vectors in anti-HIV gene therapy strategies and to express multiple proteins in single target cells, we have engineered HIV-1 vectors for the concurrent expression of multiple transgenes. Single-gene vectors, bicistronic vectors, and multigene vectors expressing up to three exogenous genes under the control of two or three different transcriptional units, placed within the viral gag-pol coding region and/or the viral nef and env genes, were designed. The genes encoding the enhanced version of green fluorescent protein (EGFP), mouse heat-stable antigen (HSA), and bacterial neomycin phosphotransferase were used as models whose expression was detected by fluorescence-activated cell sorting, fluorescence microscopy, and G418 selection. Coexpression of these reporter genes in contact-inhibited primary human skin fibroblasts (HSFs) persisted for at least 6 weeks in culture. Coexpression of the HSA and EGFP reporter genes was also achieved following cotransduction of target cells using two separate lentivirus vectors encoding HSA and EGFP, respectively. For the regulated expression of transgenes, tetracycline (Tet)-regulatable lentivirus vectors encoding the reverse Tet transactivator (rtTA) and EGFP controlled by a Tet-responsive element (TRE) were constructed. A binary HIV-1-based vector system consisting of a lentivirus encoding rtTA and a second lentivirus harboring a TRE driving the EGFP reporter gene was also designed. Doxycycline-modulated expression of the EGFP transgene was confirmed in transduced primary HSFs. These versatile vectors can potentially be used in a wide range of gene therapy applications.
Figures






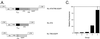
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

