Separable mechanisms of attachment and cell uptake during retrovirus infection
- PMID: 11044124
- PMCID: PMC110954
- DOI: 10.1128/jvi.74.22.10790-10795.2000
Separable mechanisms of attachment and cell uptake during retrovirus infection
Abstract
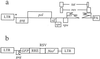
In the absence of viral envelope gene expression, cells expressing human immunodeficiency virus type 1 (HIV-1) gag and pol, accessory HIV functions, and a vector genome RNA produce and secrete large amount of noninfectious virus-like particles (VLPs) into the conditioned medium. After partial purification, such HIV-1 VLPs can be made infectious in cell-free conditions in vitro by complex formation with lipofection reagents or with the G protein of vesicular stomatitis virus (VSV-G). The resulting in vitro-modified HIV-1 particles are able to infect nondividing cells. Infectivity of envelope-free HIV VLPs can also be induced by prior modification of target cells through exposure to partially purified VSV-G vesicles. Similarly, infection can be carried out by attachment of envelope-free noninfectious VLPs to unmodified cells followed by subsequent treatment of cells with VSV-G. We interpret these findings to indicate that interaction between a viral envelope and a cell surface receptor is not necessary for the initial virus binding to the cells but is required for subsequent cell entry and infection.
Figures

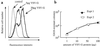

Similar articles
-
Cell surface heparan sulfate is a receptor for attachment of envelope protein-free retrovirus-like particles and VSV-G pseudotyped MLV-derived retrovirus vectors to target cells.Mol Ther. 2002 May;5(5 Pt 1):538-46. doi: 10.1006/mthe.2002.0578. Mol Ther. 2002. PMID: 11991744
-
In vitro cell-free conversion of noninfectious Moloney retrovirus particles to an infectious form by the addition of the vesicular stomatitis virus surrogate envelope G protein.J Virol. 1998 Aug;72(8):6356-61. doi: 10.1128/JVI.72.8.6356-6361.1998. J Virol. 1998. PMID: 9658075 Free PMC article.
-
Expression of human immunodeficiency virus type 1 Gag protein precursor and envelope proteins from a vesicular stomatitis virus recombinant: high-level production of virus-like particles containing HIV envelope.Virology. 2000 Mar 1;268(1):112-21. doi: 10.1006/viro.1999.0120. Virology. 2000. PMID: 10683333
-
VSV-G envelope glycoprotein forms complexes with plasmid DNA and MLV retrovirus-like particles in cell-free conditions and enhances DNA transfection.Mol Ther. 2001 Sep;4(3):232-8. doi: 10.1006/mthe.2001.0443. Mol Ther. 2001. PMID: 11545614
-
Immunogenicity and efficacy of immunodeficiency virus-like particles pseudotyped with the G protein of vesicular stomatitis virus.Virology. 2006 Jul 20;351(1):133-44. doi: 10.1016/j.virol.2006.03.009. Epub 2006 Apr 17. Virology. 2006. PMID: 16616946
Cited by
-
Synchronized infection of cell cultures by magnetically controlled virus.J Virol. 2005 Jan;79(1):622-5. doi: 10.1128/JVI.79.1.622-625.2005. J Virol. 2005. PMID: 15596857 Free PMC article.
-
Early steps of retrovirus replicative cycle.Retrovirology. 2004 May 14;1:9. doi: 10.1186/1742-4690-1-9. Retrovirology. 2004. PMID: 15169567 Free PMC article. Review.
-
Cell-cell transmission of VSV-G pseudotyped lentivector particles.PLoS One. 2013 Sep 10;8(9):e74925. doi: 10.1371/journal.pone.0074925. eCollection 2013. PLoS One. 2013. PMID: 24040363 Free PMC article.
-
Effects of polybrene and retronectin as transduction enhancers on the development and phenotypic characteristics of VHH-based CD19-redirected CAR T cells: a comparative investigation.Clin Exp Med. 2023 Oct;23(6):2535-2549. doi: 10.1007/s10238-022-00928-8. Epub 2022 Nov 25. Clin Exp Med. 2023. PMID: 36434173
-
Experimental Evolution Generates Novel Oncolytic Vesicular Stomatitis Viruses with Improved Replication in Virus-Resistant Pancreatic Cancer Cells.J Virol. 2020 Jan 17;94(3):e01643-19. doi: 10.1128/JVI.01643-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31694943 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

