Latent membrane protein 2A of Epstein-Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases
- PMID: 11046148
- PMCID: PMC102158
- DOI: 10.1128/MCB.20.22.8526-8535.2000
Latent membrane protein 2A of Epstein-Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases
Abstract
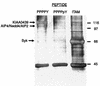
The latent membrane protein (LMP) 2A of Epstein-Barr virus (EBV) is implicated in the maintenance of viral latency and appears to function in part by inhibiting B-cell receptor (BCR) signaling. The N-terminal cytoplasmic region of LMP2A has multiple tyrosine residues that upon phosphorylation bind the SH2 domains of the Syk tyrosine kinase and the Src family kinase Lyn. The LMP2A N-terminal region also has two conserved PPPPY motifs. Here we show that the PPPPY motifs of LMP2A bind multiple WW domains of E3 protein-ubiquitin ligases of the Nedd4 family, including AIP4 and KIAA0439, and demonstrate that AIP4 and KIAA0439 form physiological complexes with LMP2A in EBV-positive B cells. In addition to a C2 domain and four WW domains, these proteins have a C-terminal Hect catalytic domain implicated in the ubiquitination of target proteins. LMP2A enhances Lyn and Syk ubiquitination in vivo in a fashion that depends on the activity of Nedd4 family members and correlates with destabilization of the Lyn tyrosine kinase. These results suggest that LMP2A serves as a molecular scaffold to recruit both B-cell tyrosine kinases and C2/WW/Hect domain E3 protein-ubiquitin ligases. This may promote Lyn and Syk ubiquitination in a fashion that contributes to a block in B-cell signaling. LMP2A may potentiate a normal mechanism by which Nedd4 family E3 enzymes regulate B-cell signaling.
Figures











References
-
- Alber G, Kim K-M, Weiser P, Riesterer C, Carsetti R, Reth M. Molecular mimicry of the antigen receptor signalling motif by transmembrane proteins of the Epstein-Barr virus and the bovine leukaemia virus. Curr Biol. 1993;3:333–339. - PubMed
-
- Beaufils P, Choquest D, Mamoun R Z, Malissen B. The (YXXL/I)2 signalling motif found in the cytoplasmic segments of the bovine leukaemia virus envelope protein and Epstein-Barr virus latent membrane protein 2A can elicit early and late lymphocyte activation events. EMBO J. 1993;12:5105–5112. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
