The distribution of heat shock proteins in the nervous system of the unstressed mouse embryo suggests a role in neuronal and non-neuronal differentiation
- PMID: 11048652
- PMCID: PMC312859
- DOI: 10.1379/1466-1268(2000)005<0291:tdohsp>2.0.co;2
The distribution of heat shock proteins in the nervous system of the unstressed mouse embryo suggests a role in neuronal and non-neuronal differentiation
Abstract
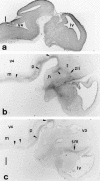
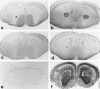
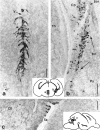
Heat shock proteins (Hsps) act as molecular chaperones and are generally constitutively expressed in the absence of stress. Hsps are also inducible by a variety of stressors whose effects could be disastrous on the brain. It has been shown previously that Hsps are differentially expressed in glial and neuronal cells, as well as in the different structures of the brain. This differential expression has been related to specific functions distinct from their general chaperone function, such as intracellular transport. We investigated here the constitutive expression of 5 Hsps (the small Hsp, Hsp25, the constitutive Hsc70 and Hsp90beta, the mainly inducible Hsp70 and Hsp90alpha), and of a molecular chaperone, TCP-1alpha during mouse nervous system development. We analyzed, by immunohistochemistry, their distribution in the central nervous system and in the ganglia of the peripheral nervous system from day 9.5 (E9.5) to day 17.5 (E17.5) of gestation. Hsps are expressed in different cell classes (neuronal, glial, and vascular). The different proteins display different but often overlapping patterns of expression in different regions of the developing nervous system, suggesting unique roles at different stages of neural maturation. Their putative function in cell remodeling during migration or differentiation and in protein transport is discussed. Moreover we consider Hsp90 function in cell signaling and the role of Hsp25 in apoptosis protection.
Figures

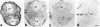




References
-
- Altman J, Bayer SA. Development of the brain stem in the rat. I. Thymidine-radiographic study of the time of origin of neurons of the lower medulla. J Comp Neurol. 1980;194:1–35. - PubMed
-
- Altman J, Bayer SA. The development of the rat hypothalamus. Adv Anat Embryol Cell Biol. 1986;100:1–178. - PubMed
-
- Altman J, Bayer SA 1995 Atlas of Prenatal Rat Brain Development. CRC Press, Boca Ràton, FL.
-
- Aquino DA, Klipfel AA, Brosnan CF, Norton WT. The 70-kDa heat shock cognate protein (HSC70) is a major constituent of the central nervous system and is up-regulated only at the mRNA level in acute experimental autoimmune encephalomyelitis. J Neurochem. 1993;61:1340–1348. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
