Phorbol esters potentiate evoked and spontaneous release by different presynaptic mechanisms
- PMID: 11050105
- PMCID: PMC6772716
- DOI: 10.1523/JNEUROSCI.20-21-07863.2000
Phorbol esters potentiate evoked and spontaneous release by different presynaptic mechanisms
Abstract
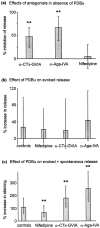
Phorbol esters enhance release from a variety of cell types. The mechanism by which phorbol esters potentiate presynaptic release from central neurons is unclear, although effects of phorbol esters both on the readily releasable pool of vesicles and on presynaptic calcium channels have been shown. Using confocal microscopy and the fluorescent styryl dye FM 1-43, we have examined the effects of phorbol-12,13-dibutyrate (PDBu) on presynaptic vesicle turnover at individually identified synapses in dissociated cultures obtained from neonatal rat hippocampus. Using different dye staining and destaining protocols we were able to resolve two effects of PDBu. Potentiation of evoked release by PDBu was insensitive to calcium channel antagonists, suggesting that this effect results from an increased number of vesicles in the readily releasable pool. Since we observed no effect of PDBu on the size of the total recycling vesicle pool, we conclude that phorbol esters alter the equilibrium between reserve and readily releasable pools. An additional effect of PDBu on spontaneous release was observed. This effect was antagonized by nifedipine but not omega-conotoxin GVIA or omega-agatoxin IVA. We conclude that PDBu influences spontaneous and evoked release by two different mechanisms: through L-type calcium channels and through an increase in the proportion of recycling vesicles in the readily releasable pool. In addition to further clarifying the mechanism of action of phorbol esters, these results suggest that phorbol esters may be a useful tool with which to probe the function of the readily releasable pool of presynaptic vesicles at CNS synapses.
Figures





References
-
- Augustin I, Rosenmund C, Südhof TC, Brose N. Munc 13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. - PubMed
-
- Betz WJ, Bewick GS. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 1992;255:200–203. - PubMed
-
- Betz WJ, Mao F, Smith CB. Imaging exocytosis and endocytosis. Curr Opin Neurobiol. 1996;6:365–371. - PubMed
-
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Südhof TC, Rettig J, Brose N. Munc 13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
