Developmental changes in synaptic AMPA and NMDA receptor distribution and AMPA receptor subunit composition in living hippocampal neurons
- PMID: 11050112
- PMCID: PMC6772733
- DOI: 10.1523/JNEUROSCI.20-21-07922.2000
Developmental changes in synaptic AMPA and NMDA receptor distribution and AMPA receptor subunit composition in living hippocampal neurons
Abstract
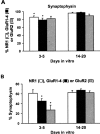
AMPA and NMDA receptors mediate most excitatory synaptic transmission in the CNS. We have developed antibodies that recognize all AMPA or all NMDA receptor variants on the surface of living neurons. AMPA receptor variants were identified with a polyclonal antibody recognizing the conserved extracellular loop region of all four AMPA receptor subunits (GluR1-4, both flip and flop), whereas NMDA receptors were immunolabeled with a polyclonal antibody that binds to an extracellular N-terminal epitope of the NR1 subunit, common to all splice variants. In non-fixed brain sections these antibodies gave labeling patterns similar to autoradiographic distributions with particularly high levels in the hippocampus. Using these antibodies, in conjunction with GluR2-specific and synaptophysin antibodies, we have directly localized and quantified surface-expressed native AMPA and NMDA receptors on cultured living hippocampal neurons during development. Using a quantitative cell ELISA, a dramatic increase was observed in the surface expression of AMPA receptors, but not NMDA receptors, between 3 and 10 d in culture. Immunocytochemical analysis of hippocampal neurons between 3 and 20 d in vitro shows no change in the proportion of synapses expressing NMDA receptors (approximately 60%) but a dramatic increase (approximately 50%) in the proportion of them that also express AMPA receptors. Furthermore, over this period the proportion of AMPA receptor-positive synapses expressing the GluR2 subunit increased from approximately 67 to approximately 96%. These changes will dramatically alter the functional properties of hippocampal synapses.
Figures








References
-
- Archibald K, Molnar E, Henley JM. Differential changes in the subcellular distribution of α-amino-3-hydroxy-5-methyl-4-isoxazole propionate and N-methyl-d-aspartate receptors in neonate and adult rat cortex. Neurosci Lett. 1999;270:49–52. - PubMed
-
- Bear MF, Abraham WC. Long-term depression in the hippocampus. Annu Rev Neurosci. 1996;19:437–462. - PubMed
-
- Bennett JA, Dingledine R. Topology profile for a glutamate receptor: three transmembrane domains and a channel-lining reentrant membrane loop. Neuron. 1995;14:373–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
