Regulation of AMPA receptor GluR1 subunit surface expression by a 4. 1N-linked actin cytoskeletal association
- PMID: 11050113
- PMCID: PMC6772741
- DOI: 10.1523/JNEUROSCI.20-21-07932.2000
Regulation of AMPA receptor GluR1 subunit surface expression by a 4. 1N-linked actin cytoskeletal association
Abstract
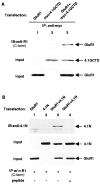
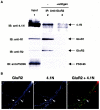
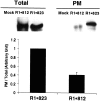
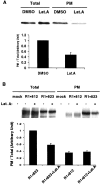
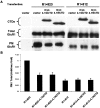
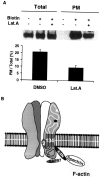
The synaptic localization, clustering, and immobilization of neurotransmitter receptors and ion channels play important roles in synapse formation and synaptic transmission. Although several proteins have been identified that interact with AMPA receptors and that may regulate their synaptic targeting, little is known about the interaction of AMPA receptors with the cytoskeleton. In studies examining the interaction of the AMPA receptor GluR1 subunit with neuronal proteins, we determined that GluR1 interacts with the 4.1G and 4.1N proteins, homologs of the erythrocyte membrane cytoskeletal protein 4.1. Using the yeast two-hybrid system and a heterologous cell system, we demonstrated that both 4.1G and 4.1N bind to a membrane proximal region of the GluR1 C terminus, and that a region within the C-terminal domain of 4.1G or 4.1N is sufficient to mediate the interaction. We also found that 4.1N can associate with GluR1 in vivo and colocalizes with AMPA receptors at excitatory synapses. Disruption of the interaction of GluR1 with 4.1N or disruption of actin filaments decreased the surface expression of GluR1 in heterologous cells. Moreover, disruption of actin filaments in cultured cortical neurons dramatically reduced the level of surface AMPA receptors. These results suggest that protein 4.1N may link AMPA receptors to the actin cytoskeleton.
Figures
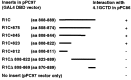


References
-
- Anderson RA, Lovrien RE. Glycophorin is linked by band 4.1 protein to the human erythrocyte membrane skeleton. Nature. 1984;307:655–658. - PubMed
-
- Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. - PubMed
-
- Blackstone CD, Levey AI, Martin LJ, Price DL, Huganir RL. Immunological detection of glutamate receptor subtypes in human central nervous system. Ann Neurol. 1992a;31:680–683. - PubMed
-
- Blackstone CD, Moss SJ, Martin LJ, Levey AI, Price DL, Huganir RL. Biochemical characterization and localization of a non-N-methyl-D-aspartate glutamate receptor in rat brain. J Neurochem. 1992b;58:1118–1126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
