In situ Ca2+ imaging reveals neurotransmitter receptors for glutamate in taste receptor cells
- PMID: 11050118
- PMCID: PMC6772752
- DOI: 10.1523/JNEUROSCI.20-21-07978.2000
In situ Ca2+ imaging reveals neurotransmitter receptors for glutamate in taste receptor cells
Abstract
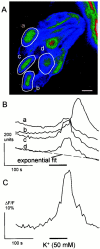
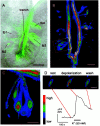
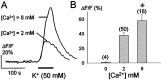
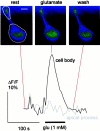
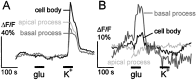
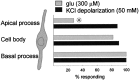
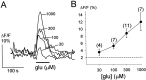
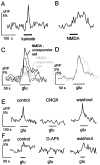
The neurotransmitters at synapses in taste buds are not yet known with confidence. Here we report a new calcium-imaging technique for taste buds that allowed us to test for the presence of glutamate receptors (GluRs) in living isolated tissue preparations. Taste cells of rat foliate papillae were loaded with calcium green dextran (CaGD). Lingual slices containing CaGD-labeled taste cells were imaged with a scanning confocal microscope and superfused with glutamate (30 micromter to 1 mm), kainate (30 and 100 micrometer), AMPA (30 and 100 micrometer), or NMDA (100 micrometer). Responses were observed in 26% of the cells that were tested with 300 micrometer glutamate. Responses to glutamate were localized to the basal processes and cell bodies, which are synaptic regions of taste cells. Glutamate responses were dose-dependent and were induced by concentrations as low as 30 microm. The non-NMDA receptor antagonists CNQX and GYKI 52466 reversibly blocked responses to glutamate. Kainate, but not AMPA, also elicited Ca(2+) responses. NMDA stimulated increases in [Ca(2+)](i) when the bath medium was modified to optimize for NMDA receptor activation. The subset of cells that responded to glutamate was either NMDA-unresponsive (54%) or NMDA-responsive (46%), suggesting that there are presumably two populations of glutamate-sensitive taste cells-one with NMDA receptors and the other without NMDA receptors. The function of GluRs in taste buds is not yet known, but the data suggest that glutamate is a neurotransmitter there. GluRs in taste cells might be presynaptic autoreceptors or postsynaptic receptors at afferent or efferent synapses.
Figures


References
-
- Akabas MH, Dodd J, Al-Awqati Q. A bitter substance induces a rise in intracellular calcium in a subpopulation of rat taste cells. Science. 1988;242:1047–1050. - PubMed
-
- Bigiani A, Delay RJ, Chaudhari N, Kinnamon SC, Roper SD. Responses to glutamate in rat taste cells. J Neurophysiol. 1997;77:3048–3059. - PubMed
-
- Bradley RM, King MS, Wang L, Shu X. Neurotransmitter and neuromodulator activity in the gustatory zone of the nucleus tractus solitarius. Chem Senses. 1996;21:377–385. - PubMed
-
- Caicedo A, Zucchi B, Roper SD. Gustatory neurons in the geniculate ganglion express AMPA and NMDA type glutamate receptors. Soc Neurosci Abstr. 1999;25:2183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
