Clathrin-mediated endocytosis near active zones in snake motor boutons
- PMID: 11050119
- PMCID: PMC6772710
- DOI: 10.1523/JNEUROSCI.20-21-07986.2000
Clathrin-mediated endocytosis near active zones in snake motor boutons
Abstract
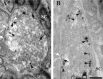
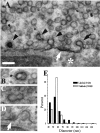
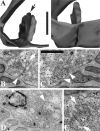
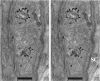
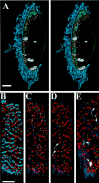
We have used the activity-dependent probe FM1-43 with electron microscopy (EM) to examine endocytosis at the vertebrate nerve-muscle synapse. Preparations were fixed after very brief neural stimulation at reduced temperature, and internalized FM1-43 was photoconverted into an electron-dense reaction product. To locate the reaction product, we reconstructed computer renderings of individual terminal boutons from serial EM sections. Most of the reaction product was seen in 40-60 nm vesicles. All of the labeled vesicles were clathrin-coated, and 92% of them were located within 300 nm of the plasma membrane, suggesting that they had undergone little processing after retrieval from their endocytic sites. The vesicles (and by inference the sites) were not dispersed randomly near the plane of the membrane but instead were clustered significantly near active zones. Additional reaction product was found within putative macropinosomes; these appeared to form from deep membrane invaginations near active zones. Thus two mechanisms of endocytosis were evident after brief stimulation. Endocytosis near active zones is consistent with the existence of local exo/endocytic cycling pools. This mechanism also might serve to maintain alignment of active zones with postsynaptic folds during periods of activity when vesicular and plasma membranes are interchanged.
Figures

Similar articles
-
Macroendocytosis and endosome processing in snake motor boutons.J Physiol. 2007 Jul 1;582(Pt 1):243-62. doi: 10.1113/jphysiol.2007.130989. Epub 2007 May 3. J Physiol. 2007. PMID: 17478535 Free PMC article.
-
Endocytic active zones: hot spots for endocytosis in vertebrate neuromuscular terminals.J Neurosci. 1999 Jun 15;19(12):4855-66. doi: 10.1523/JNEUROSCI.19-12-04855.1999. J Neurosci. 1999. PMID: 10366620 Free PMC article.
-
Effects of staurosporine on exocytosis and endocytosis at frog motor nerve terminals.J Neurosci. 2001 Feb 1;21(3):782-7. doi: 10.1523/JNEUROSCI.21-03-00782.2001. J Neurosci. 2001. PMID: 11157064 Free PMC article.
-
Monitoring synaptic vesicle recycling in frog motor nerve terminals with FM dyes.J Neurocytol. 2003 Jun-Sep;32(5-8):539-49. doi: 10.1023/B:NEUR.0000020609.19873.e8. J Neurocytol. 2003. PMID: 15034252 Review.
-
Clathrin-mediated endocytosis at the synaptic terminal: bridging the gap between physiology and molecules.Traffic. 2010 Dec;11(12):1489-97. doi: 10.1111/j.1600-0854.2010.01104.x. Traffic. 2010. PMID: 20633242 Free PMC article. Review.
Cited by
-
The spatial pattern of exocytosis and post-exocytic mobility of synaptopHluorin in mouse motor nerve terminals.J Physiol. 2009 Mar 15;587(Pt 6):1187-200. doi: 10.1113/jphysiol.2008.166728. Epub 2009 Jan 19. J Physiol. 2009. PMID: 19153160 Free PMC article.
-
Clathrin-mediated endocytosis: the physiological mechanism of vesicle retrieval at hippocampal synapses.J Physiol. 2007 Dec 15;585(Pt 3):681-6. doi: 10.1113/jphysiol.2007.139022. Epub 2007 Jun 28. J Physiol. 2007. PMID: 17599959 Free PMC article. Review.
-
Enhancement of the endosomal endocytic pathway increases quantal size.Mol Cell Neurosci. 2009 Feb;40(2):199-206. doi: 10.1016/j.mcn.2008.10.005. Epub 2008 Nov 5. Mol Cell Neurosci. 2009. PMID: 19026748 Free PMC article.
-
Macroendocytosis and endosome processing in snake motor boutons.J Physiol. 2007 Jul 1;582(Pt 1):243-62. doi: 10.1113/jphysiol.2007.130989. Epub 2007 May 3. J Physiol. 2007. PMID: 17478535 Free PMC article.
-
Endocytosis at the synaptic terminal.J Physiol. 2003 Dec 1;553(Pt 2):345-55. doi: 10.1113/jphysiol.2003.049221. Epub 2003 Sep 8. J Physiol. 2003. PMID: 12963793 Free PMC article. Review.
References
-
- Ales E, Tabares L, Poyato JM, Valero V, Lindau M, de Toledo GA. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat Cell Biol. 1999;1:40–44. - PubMed
-
- Angleson JK, Betz WJ. Monitoring secretion in real time: capacitance, amperometry, and fluorescence compared. Trends Neurosci. 1997;20:281–287. - PubMed
-
- Couteaux R, Pecot-Dechavassine M. Vesicles synaptiques et poches au niveau des “zones activies” de la jonction neuromusculaire. Comptes Rend Acad Sci Hebd Seances D (Paris) 1970;271:2346–2349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
