NMDA but not non-NMDA excitotoxicity is mediated by Poly(ADP-ribose) polymerase
- PMID: 11050121
- PMCID: PMC6772735
- DOI: 10.1523/JNEUROSCI.20-21-08005.2000
NMDA but not non-NMDA excitotoxicity is mediated by Poly(ADP-ribose) polymerase
Abstract
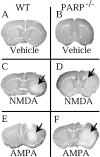
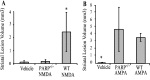
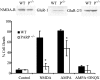
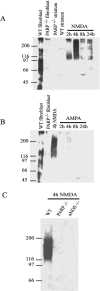
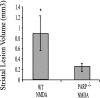
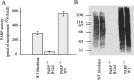
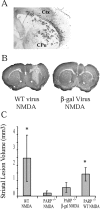
Poly(ADP-ribose) polymerase (PARP-1), a nuclear enzyme that facilitates DNA repair, may be instrumental in acute neuronal cell death in a variety of insults including, cerebral ischemia, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism, and CNS trauma. Excitotoxicity is thought to underlie these and other toxic models of neuronal death. Different glutamate agonists may trigger different downstream pathways toward neurotoxicity. We examine the role of PARP-1 in NMDA- and non-NMDA-mediated excitotoxicity. NMDA and non-NMDA agonists were stereotactically delivered into the striatum of mice lacking PARP-1 and control mice in acute (48 hr) and chronic (3 week) toxicity paradigms. Mice lacking PARP-1 are highly resistant to the excitoxicity induced by NMDA but are as equally susceptible to AMPA excitotoxicity as wild-type mice. Restoring PARP-1 protein in mice lacking PARP-1 by viral transfection restored susceptibility to NMDA, supporting the requirement of PARP-1 in NMDA neurotoxicity. Furthermore, Western blot analyses demonstrate that PARP-1 is activated after NMDA delivery but not after AMPA administration. Consistent with the theory that nitric oxide (NO) and peroxynitrite are prominent in NMDA-induced neurotoxicity, PARP-1 was not activated in mice lacking the gene for neuronal NO synthase after NMDA administration. These results suggest a selective role of PARP-1 in glutamate excitoxicity, and strategies of inhibiting PARP-1 in NMDA-mediated neurotoxicity may offer substantial acute and chronic neuroprotection.
Figures
References
-
- Affar EB, Duriez PJ, Shah RG, Sallmann FR, Bourassa S, Kupper JH, Burkle A, Poirier GG. Immunodot blot method for the detection of poly (ADP-ribose) synthesized in vitro and in vivo. Anal Biochem. 1998;259:280–283. - PubMed
-
- Affar EB, Duriez PJ, Shah RG, Winstall E, Germain M, Boucher C, Bourassa S, Kirkland JB, Poirier GG. Immunological determination and size characterization of poly(ADP- ribose) synthesized in vitro and in vivo. Biochim Biophys Acta. 1999;1428:137–146. - PubMed
-
- Beckman JS, Crow JP. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem Soc Trans. 1993;21:330–334. - PubMed
-
- Berger NA. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat Res. 1985;101:4–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
