Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment
- PMID: 11050127
- PMCID: PMC6772740
- DOI: 10.1523/JNEUROSCI.20-21-08061.2000
Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment
Abstract
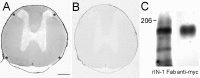
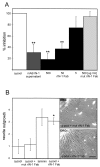
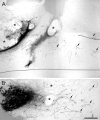
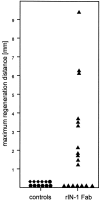
Axons in the CNS of higher vertebrates generally fail to regenerate after injury. This lack of regeneration is crucially influenced by neurite growth inhibitory protein constituents of CNS myelin. We have shown previously that a monoclonal antibody (mAb IN-1) capable of binding and neutralizing Nogo-A, a myelin-associated inhibitor of neurite growth, can induce long-distance axonal regeneration and increased structural plasticity with improved functional recovery in rat models of CNS injury. In this paper we demonstrate that a partially humanized, recombinant Fab fragment (rIN-1 Fab) derived from the original mAb IN-1, was able to promote long-distance regeneration of injured axons in the spinal cord of adult rats. When infused into a spinal cord injury site, regrowth of corticospinal fibers in 11 of 18 animals was observed after a survival time of 2 weeks. Regenerating fibers grew for >9 mm beyond the lesion site and arborized profusely in the distal cord. Regenerated fibers formed terminal arbors with varicosities in the spinal cord gray matter, strongly resembling synaptic points of contact to neurons in the spinal cord distal to the lesion. In animals that had received a bovine serum albumin solution or a recombinant IN-1 fragment that had been mutated in the antigen binding site (mutIN-1 Fab), no significant growth beyond normal lesion-induced sprouting was observed. Neutralization of endogenous nerve growth inhibitors represents a novel use of recombinant antibody technology with potential therapeutic applications after traumatic CNS lesions.
Figures





References
-
- Bandtlow C, Schiweck W, Tai HH, Schwab ME, Skerra A. The Escherichia coli-derived Fab fragment of the IgM/kappa antibody IN-1 recognizes and neutralizes myelin-associated inhibitors of neurite growth. Eur J Biochem. 1996;241:468–475. - PubMed
-
- Beattie MS, Bresnahan JC, Komon J, Tovar CA, Van Meter M, Anderson DK, Faden AI, Hsu CY, Noble LJ, Salzman S, Young W. Endogenous repair after spinal cord contusion injuries in the rat. Exp Neurol. 1997;148:453–463. - PubMed
-
- Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. - PubMed
-
- Brösamle C, Schwab ME. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J Comp Neurol. 1997;386:293–303. - PubMed
-
- Cadelli D, Schwab ME. Regeneration of lesioned septohippocampal acetylcholinesterase-positive axons is improved by antibodies against the myelin-associated neurite growth inhibitors NI-35/250. Eur J Neurosci. 1991;3:825–832. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
