2'-Hydroxylation of nicotine by cytochrome P450 2A6 and human liver microsomes: formation of a lung carcinogen precursor
- PMID: 11050152
- PMCID: PMC18791
- DOI: 10.1073/pnas.220207697
2'-Hydroxylation of nicotine by cytochrome P450 2A6 and human liver microsomes: formation of a lung carcinogen precursor
Abstract
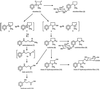
Smokers or people undergoing nicotine replacement therapy excrete approximately 10% of the nicotine dose as 4-oxo-4-(3-pyridyl)butanoic acid (keto acid) and 4-hydroxy-4-(3-pyridyl)butanoic acid (hydroxy acid). Previously, these acids were thought to arise by secondary metabolism of the major nicotine metabolite cotinine, but our data did not support this mechanism. Therefore, we hypothesized that nicotine is metabolized by 2'-hydroxylation, which would ultimately yield keto acid and hydroxy acid as urinary metabolites. This pathway had not been established previously in mammalian systems and is potentially significant because the product of nicotine 2'-hydroxylation, 4-(methylamino)-1-(3-pyridyl)-1-butanone (aminoketone), can be converted to the potent tobacco-specific lung carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Incubation of nicotine with cytochrome P450 2A6 and cofactors did indeed produce aminoketone, which was identified as its N-benzoyl derivative by GC-MS. The rate was 11% of that of cotinine production. Incubation of human liver microsomes with nicotine gave keto acid by using aminoketone as an intermediate; keto acid was not formed from cotinine. In 10 human liver samples, rates of formation of keto acid were 5.7% of those of cotinine and production of these metabolites correlated. These results provide definitive evidence for mammalian 2'-hydroxylation of nicotine and elucidate a pathway by which endogenous formation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone could occur in humans.
Figures
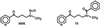
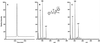
References
-
- American Cancer Society. American Cancer Society. GA: Atlanta; 2000. pp. 28–31.
-
- Gorrod J W, Schepers G. In: Analytical Determination of Nicotine and Related Compounds and Their Metabolites. Gorrod J W, Jacob P III, editors. Amsterdam: Elsevier; 1999. pp. 45–67.
-
- Nakajima M, Yamamoto T, Nunoya K-I, Yokoi T, Nagashima K, Inoue K, Funae Y, Shimada N, Kamataki T, Kuroiwa Y. Drug Metab Dispos. 1996;24:1212–1217. - PubMed
-
- Yamazaki H, Inoue K, Hashimoto M, Shimada T. Arch Toxicol. 1999;73:65–70. - PubMed
-
- Messina E S, Tyndale R F, Sellers E M. J Pharmacol Exp Ther. 1997;282:1608–1614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

