Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium
- PMID: 11050159
- PMCID: PMC18837
- DOI: 10.1073/pnas.220402097
Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium
Abstract
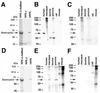
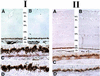
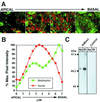
Best vitelliform macular dystrophy is a dominantly inherited, early onset, macular degenerative disease that exhibits some histopathologic similarities to age-related macular degeneration. Although the vitelliform lesion is common in the fundus of individuals with Best disease, diagnosis is based on a reduced ratio of the light peak to dark trough in the electrooculogram. Recently, the VMD2 gene on chromosome 11q13, encoding the protein bestrophin, was identified. The function of bestrophin is unknown. To facilitate studies of bestrophin, we produced both rabbit polyclonal and mouse monoclonal antibodies that proved useful for Western blotting, immunoprecipitation, and immunocytochemistry. To characterize bestrophin, we initially probed the retinal pigment epithelium (RPE)-derived cell lines ARPE-19, D407, and RPE-J. All of the cell lines expressed bestrophin mRNA by reverse transcription-PCR, but not on Western blots. Bestrophin in human RPE partitioned in the detergent phase during Triton X-114 extraction and could be modified by biotin in intact cells, indicative of a plasma membrane localization. Immunocytochemical staining of macaque and porcine eyes indicated that bestrophin is localized at the basolateral plasma membrane of RPE cells. When expressed in RPE-J cells by adenovirus-mediated gene transfer, bestrophin again was determined by confocal microscopy and cell surface biotinylation to be a basolateral plasma membrane protein. The basolateral plasma membrane localization of bestrophin suggests the possibility that bestrophin plays a role in generating the altered electrooculogram of individuals with Best disease.
Figures

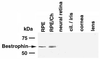

References
-
- Gass D J M. In: Stereoscopic Atlas of Macular Diseases, Diagnosis and Treatment. Gass D J M, editor. Vol. 1. St. Louis: Mosby; 1997. pp. 303–313.
-
- Marmor M F, Small K. In: The Retinal Pigment Epithelium. Wolfensberger T J, Marmor M F, editors. New York: Oxford Univ. Press; 1998. pp. 330–333.
-
- Leibowitz H M, Krueger D E, Maunder L R, Milton R C, Kini M M, Kahn H A, Nickerson R J, Pool J, Colton T L, Ganley J P, et al. Surv Ophthalmol. 1980;24:335–610. - PubMed
-
- Godel V, Chaine G, Regenbogen L, Coscas G. Acta Ophthalmol Suppl. 1986;175:1–31. - PubMed
-
- Cross H E, Bard L. Am J Ophthalmol. 1974;77:46–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

