Amplification of dopaminergic signaling by a positive feedback loop
- PMID: 11050161
- PMCID: PMC18851
- DOI: 10.1073/pnas.220410397
Amplification of dopaminergic signaling by a positive feedback loop
Abstract
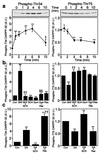
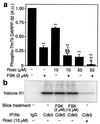
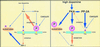
Dopamine and cAMP-regulated phosphoprotein of M(r) 32,000 (DARPP-32) plays an obligatory role in most of the actions of dopamine. In resting neostriatal slices, cyclin-dependent kinase 5 (Cdk5) phosphorylates DARPP-32 at Thr-75, thereby reducing the efficacy of dopaminergic signaling. We report here that dopamine, in slices, and acute cocaine, in whole animals, decreases the state of phosphorylation of striatal DARPP-32 at Thr-75 and thereby removes this inhibitory constraint. This effect of dopamine is achieved through dopamine D1 receptor-mediated activation of cAMP-dependent protein kinase (PKA). The activated PKA, by decreasing the state of phosphorylation of DARPP-32-Thr-75, de-inhibits itself. Dopamine D2 receptor stimulation has the opposite effect. The ability of activated PKA to reduce the state of phosphorylation of DARPP-32-Thr-75 is apparently attributable to increased protein phosphatase-2A activity, with Cdk5 being unaffected. Together, these results indicate that via positive feedback mechanisms, Cdk5 signaling and PKA signaling are mutually antagonistic.
Figures

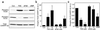

References
-
- Hemmings H C, Jr, Greengard P, Tung H Y L, Cohen P. Nature (London) 1984;310:503–505. - PubMed
-
- Greengard P, Allen P B, Nairn A C. Neuron. 1999;23:435–447. - PubMed
-
- Fienberg A A, Hiroi N, Mermelstein P, Song W-J, Snyder G L, Nishi A, Cheramy A, O'Callaghan J P, Miller D, Cole D, et al. Science. 1998;281:838–842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

