Genetic mosaic analysis based on Cre recombinase and navigated laser capture microdissection
- PMID: 11050178
- PMCID: PMC18810
- DOI: 10.1073/pnas.230237997
Genetic mosaic analysis based on Cre recombinase and navigated laser capture microdissection
Abstract
Defining molecular interactions that occur at the interface between "normal" and "abnormal" cell populations represents an important but often underexplored aspect of the pathogenesis of diseases with focal origins. Here, we illustrate an approach for conducting such analyses based on mosaic patterns of Cre recombinase expression in the adult mouse intestinal epithelium. Transgenic mice were generated that express Cre in the stem cell niche of crypts located in specified regions of their intestine. Some of these mice were engineered to allow for doxycycline-inducible Cre expression. Recombination in all pedigrees was mosaic: Cre-expressing crypts that supported recombination in all of their active multipotent stem cells were located adjacent to "control" crypts that did not express Cre at detectable levels. Cre-mediated recombination of a floxed LacZ reporter provided direct evidence that adult small-intestinal crypts contain more than one active multipotent stem cell, and that these cells can be retained in both small-intestinal and colonic crypts for at least 80 d. A method was developed to recover epithelial cells from crypts with or without recombination for subsequent gene expression profiling. Stained sections of intestine were used to create electronic image templates to guide laser capture microdissection (LCM) of adjacent frozen sections. This navigated form of LCM overcomes problems with mRNA degradation encountered when cells are marked directly by immunohistochemical methods. Combining Cre-engineered genetic mosaic mice with navigated-LCM will allow biology and pathobiology to be explored at the junction between normal and perturbed cellular cohorts.
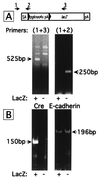
Figures





References
-
- Winton D J, Blount M A, Ponder B A J. Nature (London) 1988;333:463–466. - PubMed
-
- Schmidt G H, Winton D J, Ponder B A J. Development (Cambridge, UK) 1988;103:785–790. - PubMed
-
- Bjerknes M, Cheng H. Gastroenterology. 1999;116:7–14. - PubMed
-
- Potten C S, Loeffler M. Development (Cambridge, UK) 1990;110:1001–1020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

