"Kiss and run" exocytosis at hippocampal synapses
- PMID: 11050187
- PMCID: PMC18849
- DOI: 10.1073/pnas.230438697
"Kiss and run" exocytosis at hippocampal synapses
Abstract
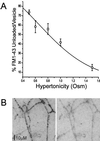
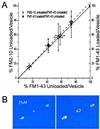
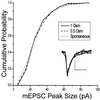
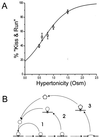
We have combined electrophysiology and imaging to measure the release of neurotransmitter and fluorescent dye at synapses of cultured hippocampal neurons. These experiments have revealed a "kiss and run" mode of exocytosis in which synaptic vesicles release glutamate normally but do not permit dye to enter or escape from the vesicle. During "kiss and run," the vesicle interior may be exposed very transiently (<6 ms), or a special configuration of the fusion pore may prevent dye exchange. We estimate that about 20% of the vesicles normally use this "kiss and run" pathway, and that the fraction of "kiss and run" events can be increased to over 80% by superfusing the synapses with hypertonic solution.
Figures
Similar articles
-
Vesicular release mode shapes the postsynaptic response at hippocampal synapses.J Physiol. 2009 Nov 1;587(Pt 21):5073-80. doi: 10.1113/jphysiol.2009.175315. Epub 2009 Sep 14. J Physiol. 2009. PMID: 19752123 Free PMC article.
-
Frequency-dependent kinetics and prevalence of kiss-and-run and reuse at hippocampal synapses studied with novel quenching methods.Neuron. 2006 Jan 19;49(2):243-56. doi: 10.1016/j.neuron.2005.12.018. Neuron. 2006. PMID: 16423698
-
Real-time three-dimensional tracking of single synaptic vesicles reveals that synaptic vesicles undergoing kiss-and-run fusion remain close to their original fusion site before reuse.Biochem Biophys Res Commun. 2019 Jun 30;514(3):1004-1008. doi: 10.1016/j.bbrc.2019.05.043. Epub 2019 May 12. Biochem Biophys Res Commun. 2019. PMID: 31092326
-
The synaptic vesicle cycle revisited.Neuron. 2000 Nov;28(2):317-20. doi: 10.1016/s0896-6273(00)00109-4. Neuron. 2000. PMID: 11144340 Review. No abstract available.
-
Regulation of exocytosis in neurons and neuroendocrine cells.Curr Opin Neurobiol. 2004 Oct;14(5):522-30. doi: 10.1016/j.conb.2004.08.008. Curr Opin Neurobiol. 2004. PMID: 15464884 Review.
Cited by
-
Presynaptic frequency- and pattern-dependent filtering.J Comput Neurosci. 2003 Sep-Oct;15(2):159-202. doi: 10.1023/a:1025812808362. J Comput Neurosci. 2003. PMID: 14512746 Review.
-
Fluorescence-based analysis of the intracytoplasmic membranes of type I methanotrophs.Microb Biotechnol. 2019 Sep;12(5):1024-1033. doi: 10.1111/1751-7915.13458. Epub 2019 Jul 1. Microb Biotechnol. 2019. PMID: 31264365 Free PMC article.
-
Synaptotagmin IV does not alter excitatory fast synaptic transmission or fusion pore kinetics in mammalian CNS neurons.J Neurosci. 2006 Jan 11;26(2):372-80. doi: 10.1523/JNEUROSCI.3997-05.2006. J Neurosci. 2006. PMID: 16407532 Free PMC article.
-
Cytosolic Accumulation of L-Proline Disrupts GABA-Ergic Transmission through GAD Blockade.Cell Rep. 2016 Oct 4;17(2):570-582. doi: 10.1016/j.celrep.2016.09.029. Cell Rep. 2016. PMID: 27705802 Free PMC article.
-
Exosome secretion kinetics are controlled by temperature.Biophys J. 2023 Apr 4;122(7):1301-1314. doi: 10.1016/j.bpj.2023.02.025. Epub 2023 Feb 22. Biophys J. 2023. PMID: 36814381 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

