Hair cell recovery in mitotically blocked cultures of the bullfrog saccule
- PMID: 11050201
- PMCID: PMC34341
- DOI: 10.1073/pnas.97.22.11722
Hair cell recovery in mitotically blocked cultures of the bullfrog saccule
Erratum in
- Proc Natl Acad Sci U S A. 2000 Dec 19;97(26):14831. Lysakowski, A [added]
Abstract
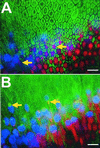
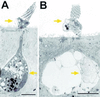
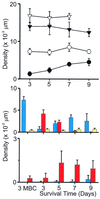
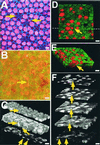
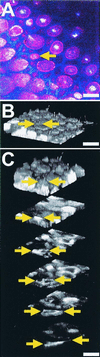
Hair cells in many nonmammalian vertebrates are regenerated by the mitotic division of supporting cell progenitors and the differentiation of the resulting progeny into new hair cells and supporting cells. Recent studies have shown that nonmitotic hair cell recovery after aminoglycoside-induced damage can also occur in the vestibular organs. Using hair cell and supporting cell immunocytochemical markers, we have used confocal and electron microscopy to examine the fate of damaged hair cells and the origin of immature hair cells after gentamicin treatment in mitotically blocked cultures of the bullfrog saccule. Extruding and fragmenting hair cells, which undergo apoptotic cell death, are replaced by scar formations. After losing their bundles, sublethally damaged hair cells remain in the sensory epithelium for prolonged periods, acquiring supporting cell-like morphology and immunoreactivity. These modes of damage appear to be mutually exclusive, implying that sublethally damaged hair cells repair their bundles. Transitional cells, coexpressing hair cell and supporting cell markers, are seen near scar formations created by the expansion of neighboring supporting cells. Most of these cells have morphology and immunoreactivity similar to that of sublethally damaged hair cells. Ultrastructural analysis also reveals that most immature hair cells had autophagic vacuoles, implying that they originated from damaged hair cells rather than supporting cells. Some transitional cells are supporting cells participating in scar formations. Supporting cells also decrease in number during hair cell recovery, supporting the conclusion that some supporting cells undergo phenotypic conversion into hair cells without an intervening mitotic event.
Figures


References
-
- Hudspeth A J. Nature (London) 1989;341:397–404. - PubMed
-
- Hudspeth A J. Neuron. 1997;19:947–950. - PubMed
-
- Corwin J T, Warchol M E. Annu Rev Neurosci. 1991;14:301–333. - PubMed
-
- Corwin J T, Warchol M E, Kelley M T. Curr Opin Neurobiol. 1993;3:32–37. - PubMed
-
- Staecker H, Van De Water T. Curr Opin Neurobiol. 1998;8:480–487. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

