Cochlear mechanisms from a phylogenetic viewpoint
- PMID: 11050203
- PMCID: PMC34343
- DOI: 10.1073/pnas.97.22.11736
Cochlear mechanisms from a phylogenetic viewpoint
Abstract
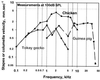
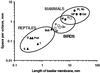
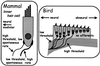
The hearing organ of the inner ear was the last of the paired sense organs of amniotes to undergo formative evolution. As a mechanical sensory organ, the inner-ear hearing organ's function depends highly on its physical structure. Comparative studies suggest that the hearing organ of the earliest amniote vertebrates was small and simple, but possessed hair cells with a cochlear amplifier mechanism, electrical frequency tuning, and incipient micromechanical tuning. The separation of the different groups of amniotes from the stem reptiles occurred relatively early, with the ancestors of the mammals branching off first, approximately 320 million years ago. The evolution of the hearing organ in the three major lines of the descendents of the stem reptiles (e.g., mammals, birds-crocodiles, and lizards-snakes) thus occurred independently over long periods of time. Dramatic and parallel improvements in the middle ear initiated papillar elongation in all lineages, accompanied by increased numbers of sensory cells with enhanced micromechanical tuning and group-specific hair-cell specializations that resulted in unique morphological configurations. This review aims not only to compare structure and function across classification boundaries (the comparative approach), but also to assess how and to what extent fundamental mechanisms were influenced by selection pressures in times past (the phylogenetic viewpoint).
Figures







References
-
- Nielsen C. Animal Evolution. Oxford: Oxford Univ. Press; 1995.
-
- Manley G A, Köppl C. Curr Opin Neurobiol. 1998;8:468–474. - PubMed
-
- Clack J A. Brain Behav Evol. 1997;50:198–212. - PubMed
-
- Carr C. In: The Evolutionary Biology of Hearing. Fay R R, Popper A N, Webster D B, editors. New York: Springer; 1992. pp. 511–543.
-
- Manley G A. In: Auditory Worlds: Sensory Analysis and Perception in Animals and Man. Manley G A, Fastl H, Kössl M, Oeckinghaus H, Klump G M, editors. Weinheim: Wiley; 2000. pp. 7–17.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

