Subdivisions of auditory cortex and processing streams in primates
- PMID: 11050211
- PMCID: PMC34351
- DOI: 10.1073/pnas.97.22.11793
Subdivisions of auditory cortex and processing streams in primates
Abstract
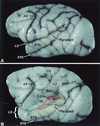
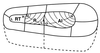
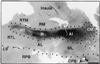
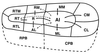
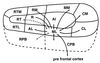
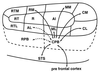
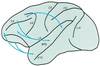
The auditory system of monkeys includes a large number of interconnected subcortical nuclei and cortical areas. At subcortical levels, the structural components of the auditory system of monkeys resemble those of nonprimates, but the organization at cortical levels is different. In monkeys, the ventral nucleus of the medial geniculate complex projects in parallel to a core of three primary-like auditory areas, AI, R, and RT, constituting the first stage of cortical processing. These areas interconnect and project to the homotopic and other locations in the opposite cerebral hemisphere and to a surrounding array of eight proposed belt areas as a second stage of cortical processing. The belt areas in turn project in overlapping patterns to a lateral parabelt region with at least rostral and caudal subdivisions as a third stage of cortical processing. The divisions of the parabelt distribute to adjoining auditory and multimodal regions of the temporal lobe and to four functionally distinct regions of the frontal lobe. Histochemically, chimpanzees and humans have an auditory core that closely resembles that of monkeys. The challenge for future researchers is to understand how this complex system in monkeys analyzes and utilizes auditory information.
Figures


References
-
- Preuss T M, Goldman-Rakic P S. J Comp Neurol. 1991;310:429–474. - PubMed
-
- Kaas J H, Krubitzer L A. In: Neuroanatomy of Visual Pathways and Their Retinotopic Organization. Dreher B, Robinson S R, editors. London: MacMillan; 1991. pp. 302–359.
-
- Walzl E M. Laryngoscope. 1947;57:778–787. - PubMed
-
- Hackett T A, Stepniewska I, Kaas J H. Brain Res. 1999;817:45–58. - PubMed
-
- Hackett T A, Stepniewska I, Kaas J H. J Comp Neurol. 1998;394:475–495. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

