Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation
- PMID: 11050244
- PMCID: PMC17322
- DOI: 10.1073/pnas.97.22.12222
Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation
Abstract
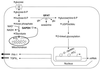
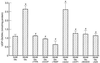
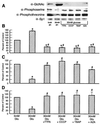
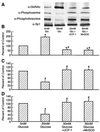
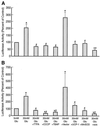
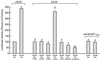
The hexosamine pathway has been implicated in the pathogenesis of diabetic complications. We determined first that hyperglycemia induced a decrease in glyceraldehyde-3-phosphate dehydrogenase activity in bovine aortic endothelial cells via increased production of mitochondrial superoxide and a concomitant 2.4-fold increase in hexosamine pathway activity. Both decreased glyceraldehyde-3-phosphate dehydrogenase activity and increased hexosamine pathway activity were prevented completely by an inhibitor of electron transport complex II (thenoyltrifluoroacetone), an uncoupler of oxidative phosphorylation (carbonyl cyanide m-chlorophenylhydrazone), a superoxide dismutase mimetic [manganese (III) tetrakis(4-benzoic acid) porphyrin], overexpression of either uncoupling protein 1 or manganese superoxide dismutase, and azaserine, an inhibitor of the rate-limiting enzyme in the hexosamine pathway (glutamine:fructose-6-phosphate amidotransferase). Immunoprecipitation of Sp1 followed by Western blotting with antibodies to O-linked GlcNAc, phosphoserine, and phosphothreonine showed that hyperglycemia increased GlcNAc by 1.7-fold, decreased phosphoserine by 80%, and decreased phosphothreonine by 70%. The same inhibitors prevented all these changes. Hyperglycemia increased expression from a transforming growth factor-beta(1) promoter luciferase reporter by 2-fold and increased expression from a (-740 to +44) plasminogen activator inhibitor-1 promoter luciferase reporter gene by nearly 3-fold. Inhibition of mitochondrial superoxide production or the glucosamine pathway prevented all these changes. Hyperglycemia increased expression from an 85-bp truncated plasminogen activator inhibitor-1 (PAI-1) promoter luciferase reporter containing two Sp1 sites in a similar fashion (3.8-fold). In contrast, hyperglycemia had no effect when the two Sp1 sites were mutated. Thus, hyperglycemia-induced mitochondrial superoxide overproduction increases hexosamine synthesis and O-glycosylation of Sp1, which activates expression of genes that contribute to the pathogenesis of diabetic complications.
Figures

References
-
- Nishikawa T, Edelstein D, Du X L, Yamagishi S I, Matsumura T, Kaneda Y, Yorek M A, Beebe D, Oates P J, Hammes H-P, et al. Nature (London) 2000;404:787–790. - PubMed
-
- National Diabetes Data Group. Diabetes in America. 2nd Ed. National Institute of Health, Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 1994.
-
- Brownlee M. Annu Rev Med. 1995;46:223–234. - PubMed
-
- Ishii H, Jirousek M R, Koya D, Takagi C, Xia P, Clermont A, Bursel S E, Kern T S, Ballas L M, Heath W F, et al. Science. 1996;272:728–731. - PubMed
-
- Park L, Raman K G, Lee K J, Lu Y, Ferran L J, Jr, Chow W S, Stern D, Schmidt A M. Nat Med. 1998;4:1025–1031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

