Etanercept (Enbrel): update on therapeutic use
- PMID: 11053088
- PMCID: PMC1766625
- DOI: 10.1136/ard.59.suppl_1.i46
Etanercept (Enbrel): update on therapeutic use
Abstract
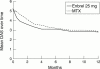
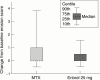
Tumour necrosis factor (TNF) is an important inflammatory disease mediator in a wide spectrum of articular diseases, including adult and juvenile rheumatoid arthritis (RA, JRA). Etanercept (Enbrel), approved in the United States and in Europe for use in patients with RA and JRA, is an effective inhibitor of TNF that has been shown to provide rapid and sustained improvement in both of these diseases. Long term studies continue to show that etanercept controls signs and symptoms of RA and JRA with no change in rate or type of adverse event over time. To demonstrate that etanercept is effective as first line treatment for patients with early active RA who have not been previously treated with methotrexate, and to examine the effect of etanercept on radiographic progression, a double blind, placebo controlled study was recently conducted, comparing etanercept with methotrexate (median dose 20 mg per week). Both etanercept 25 mg twice weekly and rapidly escalated methotrexate were effective in reducing the signs and symptoms of RA, and etanercept was significantly better than methotrexate in slowing the rate of radiographic erosions. In patients with severe psoriatic arthritis (PsA), a double blind, placebo controlled study demonstrated that etanercept was also effective in reducing disease activity in PsA. Etanercept has been well tolerated in all of these clinical trials and offers an important new treatment option to patients with inflammatory articular diseases.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

