Pharmacological examination of contractile responses of the guinea-pig isolated ileum produced by mu-opioid receptor antagonists in the presence of, and following exposure to, morphine
- PMID: 11053208
- PMCID: PMC1572416
- DOI: 10.1038/sj.bjp.0703659
Pharmacological examination of contractile responses of the guinea-pig isolated ileum produced by mu-opioid receptor antagonists in the presence of, and following exposure to, morphine
Abstract
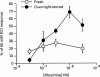
We have assessed the potential of several mu-opioid receptor antagonists to elicit a response in the guinea-pig isolated ileum in the presence of, and following overnight exposure to, morphine. Naloxone, D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH(2) (CTOP), (-)-5, 9alpha-diethyl-2-(3-furyl-methyl)-2'-hydroxy-6,7-benzomorphan (MR2266), but not D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH(2) (CTAP), produced a transient inhibition of electrically-evoked contractions of the guinea-pig ileum. The effect of 1 microM CTOP, but not that to MR2266, was inhibited by 1 microM somatostatin. Naloxone (0.3 microM), CTOP (3 microM), CTAP (3 microM) and MR2266 (0.3 microM) antagonized the inhibitory effect of morphine on electrically-evoked contractions of the guinea-pig to a similar degree and, following 60 min exposure to morphine, produced non-sustained contractions. The response to 3 microM CTOP was significantly smaller than that to 3 microM CTAP. None of the antagonists produced a response in the absence of morphine. Following overnight exposure of the ileum to 0.3 microM morphine (4 degrees C), and repeated washing to remove the agonist, all four antagonists elicited non-sustained contractions. However, the responses to 3 microM CTOP and 0.3 microM MR2266 were significantly smaller than those elicited by 0.3 microM naloxone and 3 microM CTAP. Somatostatin (1 microM) significantly reduced naloxone-induced contractions, but not those to CTAP. While all four mu-opioid antagonists elicited contractions in the presence of, and following prolonged exposure to, morphine, differences between them were noted which may be a consequence of non-opioid actions.
Figures







Similar articles
-
The mu-opioid receptor antagonist D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) [but not D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP)] produces a nonopioid receptor-mediated increase in K+ conductance of rat locus ceruleus neurons.Mol Pharmacol. 1996 Sep;50(3):650-5. Mol Pharmacol. 1996. PMID: 8794906
-
Effects of naloxone and D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 and the protein kinase inhibitors H7 and H8 on acute morphine dependence and antinociceptive tolerance in mice.J Pharmacol Exp Ther. 1996 Apr;277(1):484-90. J Pharmacol Exp Ther. 1996. PMID: 8613958
-
Selective potentiation by ouabain of naloxone-induced withdrawal contractions of isolated guinea-pig ileum following acute exposure to morphine.Br J Pharmacol. 1998 Jul;124(5):911-6. doi: 10.1038/sj.bjp.0701925. Br J Pharmacol. 1998. PMID: 9692776 Free PMC article.
-
Opioid receptor agonist potencies of morphine and morphine-6-glucuronide in the guinea-pig ileum.Eur J Pharmacol. 1994 Apr 1;255(1-3):245-7. doi: 10.1016/0014-2999(94)90105-8. Eur J Pharmacol. 1994. PMID: 8026550
-
[Characteristics and Functional Roles of Opioids Originally Present in Vivo].Yakugaku Zasshi. 2016;136(4):591-605. doi: 10.1248/yakushi.15-00265. Yakugaku Zasshi. 2016. PMID: 27040344 Review. Japanese.
Cited by
-
Neutral antagonist activity of naltrexone and 6beta-naltrexol in naïve and opioid-dependent C6 cells expressing a mu-opioid receptor.Br J Pharmacol. 2009 Apr;156(7):1044-53. doi: 10.1111/j.1476-5381.2008.00035.x. Epub 2009 Feb 13. Br J Pharmacol. 2009. PMID: 19220294 Free PMC article.
-
Citronellol, a monoterpene alcohol, reduces nociceptive and inflammatory activities in rodents.J Nat Med. 2012 Oct;66(4):637-44. doi: 10.1007/s11418-012-0632-4. Epub 2012 Feb 21. J Nat Med. 2012. PMID: 22350215
-
Endogenous kappa-opioid receptor systems inhibit hyperalgesia associated with localized peripheral inflammation.Pain. 2008 Aug 31;138(2):423-439. doi: 10.1016/j.pain.2008.01.023. Epub 2008 Mar 19. Pain. 2008. PMID: 18355964 Free PMC article.
-
The in vitro pharmacology of the peripherally restricted opioid receptor antagonists, alvimopan, ADL 08-0011 and methylnaltrexone.Naunyn Schmiedebergs Arch Pharmacol. 2007 May;375(3):205-20. doi: 10.1007/s00210-007-0146-x. Epub 2007 Mar 6. Naunyn Schmiedebergs Arch Pharmacol. 2007. PMID: 17340127
-
In vivo pharmacological resultant analysis reveals noncompetitive interactions between opioid antagonists in the rat tail-withdrawal assay.Br J Pharmacol. 2006 Dec;149(8):1071-82. doi: 10.1038/sj.bjp.0706946. Epub 2006 Oct 30. Br J Pharmacol. 2006. PMID: 17075571 Free PMC article.
References
-
- AMMER H., SCHULZ R. Chronic morphine treatment increases stimulatory Beta-2 adrenoceptor signaling in A431 cells stably expressing the Mu opioid receptor. J. Pharmacol., Exp. Ther. 1997;280:512–520. - PubMed
-
- AVIDOR-REISS T., BAYEWITCH M., LEVY R., MATUS-LEIBOVITCH N., NEVO I., VOGEL Z. Adenylyl cyclase supersensitization in μ-opioid receptor-transfected Chinese Hamster Ovary cells following chronic opioid treatment. J. Biol. Chem. 1995;270:29732–29738. - PubMed
-
- BILSKY E.J., BERNSTEIN R.N., WANG Z., SADEE W., PORRECA F. Effects of naloxone and D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 and the protein kinase inhibitors H7 and H8 on acute morphine dependence and antinociceptive tolerance in mice. J. Pharmacol. Exp. Ther. 1996;277:484–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

