Munc18c regulates insulin-stimulated glut4 translocation to the transverse tubules in skeletal muscle
- PMID: 11054418
- PMCID: PMC5540311
- DOI: 10.1074/jbc.M007419200
Munc18c regulates insulin-stimulated glut4 translocation to the transverse tubules in skeletal muscle
Abstract
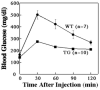
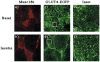
To examine the intracellular trafficking and translocation of GLUT4 in skeletal muscle, we have generated transgenic mouse lines that specifically express a GLUT4-EGFP (enhanced green fluorescent protein) fusion protein under the control of the human skeletal muscle actin promoter. These transgenic mice displayed EGFP fluorescence restricted to skeletal muscle and increased glucose tolerance characteristic of enhanced insulin sensitivity. The GLUT4-EGFP protein localized to the same intracellular compartment as the endogenous GLUT4 protein and underwent insulin- and exercise-stimulated translocation to both the sarcolemma and transverse-tubule membranes. Consistent with previous studies in adipocytes, overexpression of the syntaxin 4-binding Munc18c isoform, but not the related Munc18b isoform, in vivo specifically inhibited insulin-stimulated GLUT4-EGFP translocation. Surprisingly, however, Munc18c inhibited GLUT4 translocation to the transverse-tubule membrane without affecting translocation to the sarcolemma membrane. The ability of Munc18c to block GLUT4-EGFP translocation to the transverse-tubule membrane but not the sarcolemma membrane was consistent with substantially reduced levels of syntaxin 4 in the transverse-tubule membrane. Together, these data demonstrate that Munc18c specifically functions in the compartmentalized translocation of GLUT4 to the transverse-tubules in skeletal muscle. In addition, these results underscore the utility of this transgenic model to directly visualize GLUT4 translocation in skeletal muscle.
Figures










Similar articles
-
Regulation of insulin-stimulated GLUT4 translocation by Munc18c in 3T3L1 adipocytes.J Biol Chem. 1998 Dec 11;273(50):33876-83. doi: 10.1074/jbc.273.50.33876. J Biol Chem. 1998. PMID: 9837979
-
Inhibition of insulin-induced GLUT4 translocation by Munc18c through interaction with syntaxin4 in 3T3-L1 adipocytes.J Biol Chem. 1998 Jul 31;273(31):19740-6. doi: 10.1074/jbc.273.31.19740. J Biol Chem. 1998. PMID: 9677404
-
Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization.J Clin Invest. 2005 Feb;115(2):291-301. doi: 10.1172/JCI22681. J Clin Invest. 2005. PMID: 15690082 Free PMC article.
-
Role of SNARE's in the GLUT4 translocation response to insulin in adipose cells and muscle.J Basic Clin Physiol Pharmacol. 1998;9(2-4):153-65. doi: 10.1515/jbcpp.1998.9.2-4.153. J Basic Clin Physiol Pharmacol. 1998. PMID: 10212832 Review.
-
Intracellular organization of insulin signaling and GLUT4 translocation.Recent Prog Horm Res. 2001;56:175-93. doi: 10.1210/rp.56.1.175. Recent Prog Horm Res. 2001. PMID: 11237212 Review.
Cited by
-
Regulation of glucose transport by insulin: traffic control of GLUT4.Nat Rev Mol Cell Biol. 2012 May 23;13(6):383-96. doi: 10.1038/nrm3351. Nat Rev Mol Cell Biol. 2012. PMID: 22617471 Review.
-
Exocytosis Proteins: Typical and Atypical Mechanisms of Action in Skeletal Muscle.Front Endocrinol (Lausanne). 2022 Jun 14;13:915509. doi: 10.3389/fendo.2022.915509. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35774142 Free PMC article. Review.
-
Munc18c phosphorylation by the insulin receptor links cell signaling directly to SNARE exocytosis.J Cell Biol. 2011 Apr 4;193(1):185-99. doi: 10.1083/jcb.201007176. Epub 2011 Mar 28. J Cell Biol. 2011. PMID: 21444687 Free PMC article.
-
GLUT4 On the move.Biochem J. 2022 Feb 11;479(3):445-462. doi: 10.1042/BCJ20210073. Biochem J. 2022. PMID: 35147164 Free PMC article.
-
Dual control of cardiac Na+ Ca2+ exchange by PIP(2): electrophysiological analysis of direct and indirect mechanisms.J Physiol. 2007 Aug 1;582(Pt 3):991-1010. doi: 10.1113/jphysiol.2007.132712. Epub 2007 May 31. J Physiol. 2007. PMID: 17540705 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

