PCR bias in ecological analysis: a case study for quantitative Taq nuclease assays in analyses of microbial communities
- PMID: 11055948
- PMCID: PMC92404
- DOI: 10.1128/AEM.66.11.4945-4953.2000
PCR bias in ecological analysis: a case study for quantitative Taq nuclease assays in analyses of microbial communities
Abstract
Succession of ecotypes, physiologically diverse strains with negligible rRNA sequence divergence, may explain the dominance of small, red-pigmented (phycoerythrin-rich) cyanobacteria in the autotrophic picoplankton of deep lakes (C. Postius and A. Ernst, Arch. Microbiol. 172:69-75, 1999). In order to test this hypothesis, it is necessary to determine the abundance of specific ecotypes or genotypes in a mixed background of phylogenetically similar organisms. In this study, we examined the performance of Taq nuclease assays (TNAs), PCR-based assays in which the amount of an amplicon is monitored by hydrolysis of a labeled oligonucleotide (TaqMan probe) when hybridized to the amplicon. High accuracy and a 7-order detection range made the real-time TNA superior to the corresponding end point technique. However, in samples containing mixtures of homologous target sequences, quantification can be biased due to limited specificity of PCR primers and probe oligonucleotides and due to accumulation of amplicons that are not detected by the TaqMan probe. A decrease in reaction efficiency, which can be recognized by direct monitoring of amplification, provides experimental evidence for the presence of such a problem and emphasizes the need for real-time technology in quantitative PCR. Use of specific primers and probes and control of amplification efficiency allow correct quantification of target DNA in the presence of an up to 10(4)-fold excess of phylogenetically similar DNA and of an up to 10(7)-fold excess of dissimilar DNA.
Figures


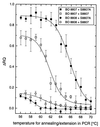


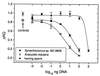



References
-
- Albis-Camps M, Blasczyk R. Fluorotyping of HLA-DRB by sequence-specific priming and fluorogenic probing. Tissue Antigens. 1999;53:301–307. - PubMed
-
- Assmann D. Nahrungsselektion und Nahrungsverwertung chroococcaler Cyanobakterien durch heterotrophe Nanoflagellaten. Ph.D. thesis. Constance, Germany: Universität Konstanz; 1998.
-
- Beard S J, Handley B A, Hayes P K, Walsby A E. The diversity of gas vesicle genes in Planktothrix rubescens from Lake Zurich. Microbiology. 1999;145:2757–2768. - PubMed
-
- Bright D I, Walsby A E. The relationship between critical pressure and width of gas vesicles in isolates of Planktothrix rubescens from Lake Zurich. Microbiology. 1999;145:2769–2775. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

