Comparison of acid mine drainage microbial communities in physically and geochemically distinct ecosystems
- PMID: 11055950
- PMCID: PMC92406
- DOI: 10.1128/AEM.66.11.4962-4971.2000
Comparison of acid mine drainage microbial communities in physically and geochemically distinct ecosystems
Abstract
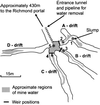
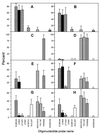
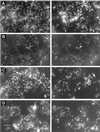
This study presents population analyses of microbial communities inhabiting a site of extreme acid mine drainage (AMD) production. The site is the inactive underground Richmond mine at Iron Mountain, Calif., where the weathering of a massive sulfide ore body (mostly pyrite) produces solutions with pHs of approximately 0.5 to approximately 1.0. Here we used a suite of oligonucleotide probes, designed from molecular data recently acquired from the site, to analyze a number of microbial environments by fluorescent in situ hybridization. Microbial-community analyses were correlated with geochemical and mineralogical data from those environments. The environments investigated were within the ore body and thus at the site of pyrite dissolution, as opposed to environments that occur downstream of the dissolution. Few organism types, as defined by the specificities of the oligonucleotide probes, dominated the microbial communities. The majority of the dominant organisms detected were newly discovered or organisms only recently associated with acid-leaching environments. "Ferroplasma" spp. were detected in many of the communities and were particularly dominant in environments of lowest pH and highest ionic strength. Leptospirillum spp. were also detected in many slime and pyrite-dominated environments. In samples of an unusual subaerial slime, a new uncultured Leptospirillum sp. dominated. Sulfobacillus spp. were detected as a prominent inhabitant in warmer ( approximately 43 degrees C) environments. The information gathered here is critical for determining organisms important to AMD production at Iron Mountain and for directing future studies of this process. The findings presented here also have relevance to the microbiology of industrial bioleaching and to the understanding of geochemical iron and sulfur cycles.
Figures



References
-
- Adams M W W. Size limits of very small microorganisms. Washington, D.C.: National Academy Press; 1999. The influence of environment and metabolic capacity on the size of a microorganism; pp. 74–80.
-
- Atlas R M, Horowitz A, Krichevsky M, Bej A K. Response of microbial-populations to environmental disturbance. Microb Ecol. 1991;22:249–256. - PubMed
-
- Blake R C, Shute E A, Greenwood M M, Spencer G H, Ingledew W J. Enzymes of aerobic respiration on iron. FEMS Microbiol Rev. 1993;11:9–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

