Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis
- PMID: 11056663
- PMCID: PMC17779
- DOI: 10.1186/ar14
Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis
Abstract
Introduction: Rheumatoid arthritis (RA) is associated with an increased production of a range of cytokines including tumour necrosis factor (TNF)-alpha and interleukin (IL)-1, which display potent proinflammatory actions that are thought to contribute to the pathogenesis of the disease. Although TNF-alpha seems to be the major cytokine in the inflammatory process, IL-1 is the key mediator with regard to cartilage and bone destruction. Apart from direct blockage of IL-1/TNF, regulation can be exerted at the level of modulatory cytokines such as IL-1 and IL-10. IL-4 is a pleiotropic T-cell derived cytokine that can exert either suppressive or stimulatory effects on different cell types, and was originally identified as a B-cell growth factor and regulator of humoral immune pathways. IL-4 is produced by activated CD4+T cells and it promotes the maturation of TH2 cells. IL-4 stimulates proliferation, differentiation and activation of several cell types, including fibroblasts, endothelial cells and epithelial cells. IL-4 is also known to be a potent anti-inflammatory cytokine that acts by inhibiting the synthesis of proinflammatory cytokines such as IL-1, TNF-alpha, IL-6, IL-8 and IL-12 by macrophages and monocytes. Moreover, IL-4 stimulates the synthesis of several cytokine inhibitors such as interleukin-1 receptor antagonist (IL-1Ra), soluble IL-1-receptor type II and TNF receptors IL-4 suppresses metalloproteinase production and stimulates tissue inhibitor of metalloproteinase-1 production in human mononuclear phagocytes and cartilage explants, indicating a protective effect of IL-4 towards extracellular matrix degradation. Furthermore, IL-4 inhibits both osteoclast activity and survival, and thereby blocks bone resorption in vitro. Of great importance is that IL-4 could not be detected in synovial fluid or in tissues. This absence of IL-4 in the joint probably contributes to the disturbance in the Th1/Th2 balance in chronic RA. Collagen-induced arthritis (CIA) is a widely used model of arthritis that displays several features of human RA. Recently it was demonstrated that the onset of CIA is under stringent control of IL-4 and IL-10. Furthermore, it was demonstrated that exposure to IL-4 during the immunization stage reduced onset and severity of CIA. However, after cessation of IL-4 treatment disease expression increased to control values.
Aims: Because it was reported that IL-4 suppresses several proinflammatory cytokines and matrix degrading enzymes and upregulates inhibitors of both cytokines and catabolic enzymes, we investigated the tissue protective effect of systemic IL-4 treatment using established murine CIA as a model. Potential synergy of low dosages of anti-inflammatory glucocorticosteroids and IL-4 was also evaluated.
Methods: DBA-1J/Bom mice were immunized with bovine type II collagen and boosted at day 21. Mice with established CIA were selected at day 28 after immunization and treated for days with IL-4, prednisolone, or combinations of prednisolone and IL-4. Arthritis score was monitored visually. Joint pathology was evaluated by histology, radiology and serum cartilage oligomeric matrix protein (COMP). In addition, serum levels of IL-1Ra and anticollagen antibodies were determined.
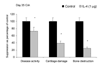
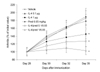
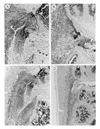
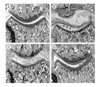
Results: Treatment of established CIA with IL-4 (1microgram/day) resulted in suppression of disease activity as depicted in Figure 1. Of great interest is that, although 1 microgram/day IL-4 had only a moderate effect on the inflammatory component of the disease activity, it strongly reduced cartilage pathology, as determined by histological examination (Fig. 1). Moreover, serum COMP levels were significantly reduced, confirming decreased cartilage involvement. In addition, both histological and radiological analysis showed that bone destruction was prevented (Fig. 1). Systemic IL-4 administration increased serum IL-1Ra levels and reduced anticollagen type II antibody levels. Treatment with low-dose IL-4 (0.1 microgram/day) was ineffective in suppressing disease score, serum COMP or joint destruction. Synergistic suppression of both arthritis severity and COMP levels was noted when low-dose IL-4 was combined with prednisolone (0.05 mg/kg/day), however, which in itself was not effective.
Discussion: In the present study, we demonstrate that systemic IL-4 treatment ameliorates disease progression of established CIA. Although clinical disease progression of established CIA. Although clinical disease progression was only arrested and not reversed, clear protection against cartilage and bone destruction was noted. This is in accord with findings in both human RA and animal models of RA that show that inflammation and tissue destruction sometimes are uncoupled processes. Of great importance is that, although inflammation was still present, strong reduction in serum COMP was found after exposure to IL-4. This indicated that serum COMP levels reflected cartilage damage, although a limited contribution of the inflamed synovium cannot be excluded. Increased serum IL-1Ra level (twofold) was found after systemic treatment with IL-4, but it is not likely that this could explain the suppression of CIA. We and others have reported that high dosages of IL-1Ra are needed for marked suppression of CIA. As reported previously, lower dosages of IL-4 did not reduce clinical disease severity of established CIA. Of importance is that combined treatment of low dosages of IL-4 and IL-10 appeared to have more potent anti-inflammatory effects, and markedly protected against cartilage destruction. Improved anti-inflammatory effect was achieved with IL-4/prednisolone treatment. In addition, synergistic effects were found for the reduction of cartilage and bone destruction. This indicates that systemic IL-4/prednisolone treatment may provide a cartilage and bone protective therapy for human RA.
Figures




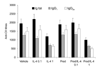
References
-
- Mosmann TR, Coffman RL. Th1 and Th2 cells: different pattern of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. - PubMed
-
- Paul WE, Ohara J. B-cell stimulatory factor-1/interleukin-4. . Annu Rev Immunol. 1987;5:429–459. - PubMed
-
- Chomarat P, Banchereau J. An update on interleukin-4 and its receptor. Eur Cytokine Net. 1997;8:333–344. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

